Age and association of kidney measures with mortality and end-stage renal disease - PubMed (original) (raw)
Meta-Analysis
. 2012 Dec 12;308(22):2349-60.
doi: 10.1001/jama.2012.16817.
Kunihiro Matsushita, Yingying Sang, Bakhtawar K Mahmoodi, Corri Black, Areef Ishani, Nanne Kleefstra, David Naimark, Paul Roderick, Marcello Tonelli, Jack F M Wetzels, Brad C Astor, Ron T Gansevoort, Adeera Levin, Chi-Pang Wen, Josef Coresh; Chronic Kidney Disease Prognosis Consortium
Collaborators, Affiliations
- PMID: 23111824
- PMCID: PMC3936348
- DOI: 10.1001/jama.2012.16817
Meta-Analysis
Age and association of kidney measures with mortality and end-stage renal disease
Stein I Hallan et al. JAMA. 2012.
Abstract
Context: Chronic kidney disease (CKD) is prevalent in older individuals, but the risk implications of low estimated glomerular filtration rate (eGFR) and high albuminuria across the full age range are controversial.
Objective: To evaluate possible effect modification (interaction) by age of the association of eGFR and albuminuria with clinical risk, examining both relative and absolute risks.
Design, setting, and participants: Individual-level meta-analysis including 2,051,244 participants from 33 general population or high-risk (of vascular disease) cohorts and 13 CKD cohorts from Asia, Australasia, Europe, and North/South America, conducted in 1972-2011 with a mean follow-up time of 5.8 years (range, 0-31 years).
Main outcome measures: Hazard ratios (HRs) of mortality and end-stage renal disease (ESRD) according to eGFR and albuminuria were meta-analyzed across age categories after adjusting for sex, race, cardiovascular disease, diabetes, systolic blood pressure, cholesterol, body mass index, and smoking. Absolute risks were estimated using HRs and average incidence rates.
Results: Mortality (112,325 deaths) and ESRD (8411 events) risks were higher at lower eGFR and higher albuminuria in every age category. In general and high-risk cohorts, relative mortality risk for reduced eGFR decreased with increasing age; eg, adjusted HRs at an eGFR of 45 mL/min/1.73 m2 vs 80 mL/min/1.73 m2 were 3.50 (95% CI, 2.55-4.81), 2.21 (95% CI, 2.02-2.41), 1.59 (95% CI, 1.42-1.77), and 1.35 (95% CI, 1.23-1.48) in age categories 18-54, 55-64, 65-74, and ≥75 years, respectively (P <.05 for age interaction). Absolute risk differences for the same comparisons were higher at older age (9.0 [95% CI, 6.0-12.8], 12.2 [95% CI, 10.3-14.3], 13.3 [95% CI, 9.0-18.6], and 27.2 [95% CI, 13.5-45.5] excess deaths per 1000 person-years, respectively). For increased albuminuria, reduction of relative risk with increasing age was less evident, while differences in absolute risk were higher in older age categories (7.5 [95% CI, 4.3-11.9], 12.2 [95% CI, 7.9-17.6], 22.7 [95% CI, 15.3-31.6], and 34.3 [95% CI, 19.5-52.4] excess deaths per 1000 person-years, respectively by age category, at an albumin-creatinine ratio of 300 mg/g vs 10 mg/g). In CKD cohorts, adjusted relative hazards of mortality did not decrease with age. In all cohorts, ESRD relative risks and absolute risk differences at lower eGFR or higher albuminuria were comparable across age categories.
Conclusions: Both low eGFR and high albuminuria were independently associated with mortality and ESRD regardless of age across a wide range of populations. Mortality showed lower relative risk but higher absolute risk differences at older age.
Conflict of interest statement
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. None of the authors have conflicts influencing this analysis.
Figures
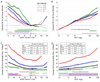
Figure 1
Adjusted hazard ratios (panels A and B) for all-cause mortality and average mortality rate (panels C and D) according to eGFR (A and C) and ACR (B and D) within each age category. The filled circles note statistical significance (p<0.05) compared to the reference (black diamond) eGFR of 80 ml/min1.73m2 or ACR of 10 mg/g within each age category in panels A and B and compared to age category 55–64 years in panels C and D. Plus signs and open circles on the bottom of each panel represent significantly positive (greater effect size) and negative (smaller effect size) point-wise interactions (p<0.05), respectively, compared to age 55–64 years. Gaps indicate no significant point-wise interaction. Models are meta-analysis of general population and high risk cohorts adjusted for sex, race, body mass index, systolic blood pressure, total cholesterol, history of cardiovascular disease, diabetes, smoking status, and albuminuria (panels A and C) or eGFR (panels B and D).
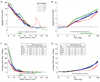
Figure 2
Adjusted hazard ratios (panels A and B) and average incidence rate (panels C and D) for ESRD according to eGFR (A and C) and ACR (B and D) within each age category. The filled circles note statistical significance (p<0.05) compared to the reference (black diamond) eGFR of 80 ml/min1.73m2 or ACR of 10 mg/g within each age category in panels A and B and compared to age category 55–64 years in panels C and D. Plus signs and open circles on the bottom of each panel represent significantly positive (greater effect size) and negative (smaller effect size) point-wise interactions (p<0.05), respectively, compared to age 55–64 years. Gaps indicate no significant point-wise interaction. Models are meta-analysis of general population and high risk cohorts adjusted for sex, race, body mass index, systolic blood pressure, total cholesterol, history of cardiovascular disease, diabetes, smoking status, and albuminuria (panels A and C) or eGFR (panels B and D).
Figure 3
Adjusted hazard ratio (panel A) and incidence rate difference (panel B) for all-cause mortality by categories of eGFR and albuminuria across age groups. Each number represents a pooled estimate from meta-analysis in 34 general population and high risk cohorts. All results are adjusted for covariates. Bold numbers indicated statistical significance at P<0.05 compared to the reference cell and circles (○) indicate a significant negative interaction and stars (*) indicate a significant positive interaction at P<0.05 compared to the corresponding cell in the 55–64 year age group. Color shading indicates the strength of association (approximately one quarter of all cells are shaded in each color; Green: low; yellow: mild; orange: moderate; red: high) measured as either hazard ratios (panel A) or incidence rate difference (panel B). Confidence intervals are provided in eTable 2.
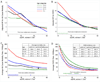
Figure 4
Adjusted hazard ratios (panels A and B) and average incidence rate (panels C and D) for all-cause mortality (A and C) and ESRD (B and D) in CKD cohorts according to eGFR within each age category. The filled circles note statistical significance (p<0.05) compared to the reference (black diamond) eGFR of 50 ml/min1.73m2 within each age category in panels A and B and compared to age category 55–64 years in panels C and D. Plus signs and open circles on the bottom of each panel represent significantly positive (greater effect size) and negative (smaller effect size) point-wise interactions (p<0.05), respectively, compared to age 55–64 years. Gaps indicate no significant point-wise interaction. Models are meta-analysis of CKD cohorts adjusted for sex, race, body mass index, systolic blood pressure, total cholesterol, history of cardiovascular disease, diabetes, smoking status, and albuminuria.
Comment in
- Chronic kidney disease—a challenge for all ages.
de Boer IH. de Boer IH. JAMA. 2012 Dec 12;308(22):2401-2. doi: 10.1001/jama.2012.30761. JAMA. 2012. PMID: 23111858 No abstract available. - Chronic kidney disease: The effect of age on CKD outcomes.
Allison SJ. Allison SJ. Nat Rev Nephrol. 2013 Jan;9(1):3. doi: 10.1038/nrneph.2012.256. Epub 2012 Nov 20. Nat Rev Nephrol. 2013. PMID: 23165297 No abstract available. - [Chronic kidney disease: how high are the risks?--The progression of chronic kidney disease has to be influenced].
Alscher MD. Alscher MD. Dtsch Med Wochenschr. 2013 Mar;138(10):462. doi: 10.1055/s-0032-1329037. Epub 2013 Feb 26. Dtsch Med Wochenschr. 2013. PMID: 23444019 German. No abstract available. - Measures to define chronic kidney disease.
O'Hare AM. O'Hare AM. JAMA. 2013 Apr 3;309(13):1343. doi: 10.1001/jama.2013.1328. JAMA. 2013. PMID: 23549569 No abstract available. - Measures to define chronic kidney disease--reply.
Hallan SI, Matsushita K, Coresh J; Chronic Kidney Disease Prognosis Consortium. Hallan SI, et al. JAMA. 2013 Apr 3;309(13):1343-4. doi: 10.1001/jama.2013.1349. JAMA. 2013. PMID: 23549570 No abstract available. - Age and outcomes in CKD.
Tangri N, Komenda P, Rigatto C. Tangri N, et al. Am J Kidney Dis. 2013 Aug;62(2):225-7. doi: 10.1053/j.ajkd.2013.03.012. Epub 2013 Apr 20. Am J Kidney Dis. 2013. PMID: 23608544 No abstract available.
Similar articles
- Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis.
Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium. Fox CS, et al. Lancet. 2012 Nov 10;380(9854):1662-73. doi: 10.1016/S0140-6736(12)61350-6. Epub 2012 Sep 24. Lancet. 2012. PMID: 23013602 Free PMC article. Review. - Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis.
Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, Yamashita K, Zhang L, Coresh J, de Jong PE, Astor BC; Chronic Kidney Disease Prognosis Consortium. Mahmoodi BK, et al. Lancet. 2012 Nov 10;380(9854):1649-61. doi: 10.1016/S0140-6736(12)61272-0. Epub 2012 Sep 24. Lancet. 2012. PMID: 23013600 Free PMC article. Review. - Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts.
Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium; Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Astor BC, et al. Kidney Int. 2011 Jun;79(12):1331-40. doi: 10.1038/ki.2010.550. Epub 2011 Feb 2. Kidney Int. 2011. PMID: 21289598 Free PMC article. - Estimated Glomerular Filtration Rate, Albuminuria, and Adverse Outcomes: An Individual-Participant Data Meta-Analysis.
Writing Group for the CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K, Ballew SH, Sang Y, Surapaneni A, Alencar de Pinho N, Anderson A, Appel LJ, Ärnlöv J, Azizi F, Bansal N, Bell S, Bilo HJG, Brunskill NJ, Carrero JJ, Chadban S, Chalmers J, Chen J, Ciemins E, Cirillo M, Ebert N, Evans M, Ferreiro A, Fu EL, Fukagawa M, Green JA, Gutierrez OM, Herrington WG, Hwang SJ, Inker LA, Iseki K, Jafar T, Jassal SK, Jha V, Kadota A, Katz R, Köttgen A, Konta T, Kronenberg F, Lee BJ, Lees J, Levin A, Looker HC, Major R, Melzer Cohen C, Mieno M, Miyazaki M, Moranne O, Muraki I, Naimark D, Nitsch D, Oh W, Pena M, Purnell TS, Sabanayagam C, Satoh M, Sawhney S, Schaeffner E, Schöttker B, Shen JI, Shlipak MG, Sinha S, Stengel B, Sumida K, Tonelli M, Valdivielso JM, van Zuilen AD, Visseren FLJ, Wang AY, Wen CP, Wheeler DC, Yatsuya H, Yamagata K, Yang JW, Young A, Zhang H, Zhang L, Levey AS, Gansevoort RT. Writing Group for the CKD Prognosis Consortium, et al. JAMA. 2023 Oct 3;330(13):1266-1277. doi: 10.1001/jama.2023.17002. JAMA. 2023. PMID: 37787795 Free PMC article. - Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality.
Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS. Coresh J, et al. JAMA. 2014 Jun 25;311(24):2518-2531. doi: 10.1001/jama.2014.6634. JAMA. 2014. PMID: 24892770 Free PMC article.
Cited by
- Long-Term Follow-Up of Patients with Chronic Kidney Disease: Renal Function Decline and Associated Risk Factors.
Issac AS, Singh S, Imran R, Shashank C, Kashif H, Sheikh Z, Dutta P. Issac AS, et al. J Pharm Bioallied Sci. 2024 Jul;16(Suppl 3):S2431-S2433. doi: 10.4103/jpbs.jpbs_231_24. Epub 2024 Jul 31. J Pharm Bioallied Sci. 2024. PMID: 39346205 Free PMC article. - Urinary extracellular vesicles as a monitoring tool for renal damage in patients not meeting criteria for chronic kidney disease.
Anfaiha-Sanchez M, Santiago-Hernandez A, Lopez JA, Lago-Baameiro N, Pardo M, Martin-Blazquez A, Vazquez J, Ruiz-Hurtado G, Barderas MG, Segura J, Ruilope LM, Martin-Lorenzo M, Alvarez-Llamas G. Anfaiha-Sanchez M, et al. J Extracell Biol. 2024 Sep 17;3(9):e170. doi: 10.1002/jex2.170. eCollection 2024 Sep. J Extracell Biol. 2024. PMID: 39290459 Free PMC article. - The Urinary Glycopeptide Profile Differentiates Early Cardiorenal Risk in Subjects Not Meeting Criteria for Chronic Kidney Disease.
Santiago-Hernandez A, Martin-Lorenzo M, Gómez-Serrano M, Lopez JA, Martin-Blazquez A, Vellosillo P, Minguez P, Martinez PJ, Vázquez J, Ruiz-Hurtado G, Barderas MG, Sarafidis P, Segura J, Ruilope LM, Alvarez-Llamas G. Santiago-Hernandez A, et al. Int J Mol Sci. 2024 Jun 26;25(13):7005. doi: 10.3390/ijms25137005. Int J Mol Sci. 2024. PMID: 39000114 Free PMC article. - Treatment of chronic kidney disease in older populations.
Kishi S, Kadoya H, Kashihara N. Kishi S, et al. Nat Rev Nephrol. 2024 Sep;20(9):586-602. doi: 10.1038/s41581-024-00854-w. Epub 2024 Jul 8. Nat Rev Nephrol. 2024. PMID: 38977884 Review. - Metabolic syndrome as a risk factor for the development of kidney dysfunction: a meta-analysis of observational cohort studies.
Valizadeh A, Nikoohemmat M, Ebadinejad A, Soltani S, Tape PMK, Sohrabi A, Abiri B, Valizadeh M. Valizadeh A, et al. J Diabetes Metab Disord. 2023 Nov 23;23(1):215-227. doi: 10.1007/s40200-023-01348-5. eCollection 2024 Jun. J Diabetes Metab Disord. 2023. PMID: 38932881 Review.
References
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–S266. - PubMed
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. - PubMed
- Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006 Aug;17(8):2275–2284. - PubMed
- Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51(3):373–384. - PubMed
- Gansevoort RT, de Jong PE. The Case for Using Albuminuria in Staging Chronic Kidney Disease. J Am Soc Nephrol. 2009;20(3):465–468. - PubMed
Publication types
MeSH terms
Grants and funding
- N01 HC085086/HC/NHLBI NIH HHS/United States
- U01 DK035073/DK/NIDDK NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- N01 HC075150/HC/NHLBI NIH HHS/United States
- N01 HC025195/HC/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- N01HC55222/HL/NHLBI NIH HHS/United States
- R01 AG007181/AG/NIA NIH HHS/United States
- R01 DK073217/DK/NIDDK NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- N01 HC095169/HC/NHLBI NIH HHS/United States
- R01 HL080295/HL/NHLBI NIH HHS/United States
- R01 AG020098/AG/NIA NIH HHS/United States
- N01 HC035129/HC/NHLBI NIH HHS/United States
- N01 HC095159/HC/NHLBI NIH HHS/United States
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- K23 DK067303/DK/NIDDK NIH HHS/United States
- R01 AG015928/AG/NIA NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- K23 DK002904/DK/NIDDK NIH HHS/United States
- U10 EY006594/EY/NEI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- N01 HC015103/HC/NHLBI NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- R01 DK031801/DK/NIDDK NIH HHS/United States
- U01 NS041588/NS/NINDS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- N01 HC085079/HC/NHLBI NIH HHS/United States
- R01 HL068140/HL/NHLBI NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- R01 AG028507/AG/NIA NIH HHS/United States
- R01 AG027058/AG/NIA NIH HHS/United States
- N01 HC045133/HC/NHLBI NIH HHS/United States
- CZH/4/656/CSO_/Chief Scientist Office/United Kingdom
- R01 HL043232-03/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous