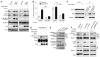Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation - PubMed (original) (raw)
Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation
Richard C Wang et al. Science. 2012.
Abstract
Aberrant signaling through the class I phosphatidylinositol 3-kinase (PI3K)-Akt axis is frequent in human cancer. Here, we show that Beclin 1, an essential autophagy and tumor suppressor protein, is a target of the protein kinase Akt. Expression of a Beclin 1 mutant resistant to Akt-mediated phosphorylation increased autophagy, reduced anchorage-independent growth, and inhibited Akt-driven tumorigenesis. Akt-mediated phosphorylation of Beclin 1 enhanced its interactions with 14-3-3 and vimentin intermediate filament proteins, and vimentin depletion increased autophagy and inhibited Akt-driven transformation. Thus, Akt-mediated phosphorylation of Beclin 1 functions in autophagy inhibition, oncogenesis, and the formation of an autophagy-inhibitory Beclin 1/14-3-3/vimentin intermediate filament complex. These findings have broad implications for understanding the role of Akt signaling and intermediate filament proteins in autophagy and cancer.
Figures
Fig. 1
Akt suppression of autophagy, interaction with Beclin 1, and phosphorylation of Beclin 1. (A) Biochemical assessment of autophagy (p62 and LC3) and mTOR activity (p-4E-BP1) in HeLa cells expressing constitutively active Akt (myr-Akt1) or control vector, grown in normal medium or starved in Earles’ Balance Salt Solution (EBSS) for 2 hours, and treated with 250 nM Torin 1 or control solution. (B) GFP-LC3 dots (autophagosomes) in HeLa/GFP-LC3 cells treated as in (A). Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (C) Immunoprecipitation of endogenous Akt with endogenous Beclin 1 in HeLa cells with or without starvation for 2 hours. (D) In vitro phosphorylation of Flag-Beclin 1 S295 by GST-Akt1 with or without 1 μM indicated Akt inhibitors. (E) Phosphorylation of endogenous Beclin 1 S295 and Beclin 1 S234 in HeLa cells transfected with control vector or HA-myr-Akt1 with or without Torin1 for 4 hours. (E) Phosphorylation of endogenous Beclin 1 S295 in paired melanoma (WM793 and 451Lu), glioblastoma (U87-MG, U87-MG + PTEN), and breast cancer (MCF-10DCIS and MDMB-231) cells with high and low activities of Akt, respectively. *P<0.05; **P<0.01; Tukey test. WCL, whole cell lysates.
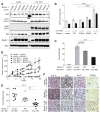
Fig. 2
Effect of a non-phosphorylatable mutant of Beclin 1 (Beclin 1 S234A/S295A (AA)) on autophagy, anchorage-independent growth, and Akt-mediated tumorigenesis. (A) Biochemical assessment of autophagy in Rat2 fibroblasts expressing indicated Flag-Beclin 1 and Akt constructs. (B) GFP-LC3 dots in Rat2 cells transduced with indicated lentiviral vectors and transfected with GFP-LC3. Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (C) Soft agar colonies formed by Rat2 fibroblasts transduced with indicated vectors. Bars represent mean ± SEM of triplicate samples of ≥12 random images analyzed by ImageJ. Similar results observed in 3 independent experiments. (D) Xenograft tumor volumes formed by Rat2 fibroblasts transduced with indicated vectors. (P<0.001 for myr-Akt1 + Beclin 1 AA group vs. myr-Akt1 + vector or myr-Akt1 + Beclin 1 groups; linear-mixed effect model). (E) Tumor weights at day 21. (F) Representative images of tumors formed by Rat2 fibroblasts. Arrows denote representative p62-, Ki-67-, and TUNEL-positive cells. Scale bars, 20 μm. For (D) and (E), results represent mean ± SEM for all tumors in each genotype (9–10 per group). *P<0.05, **P<0.01, ***P<0.001; Tukey-test.
Fig. 3
Interactions between Beclin 1 and 14-3-3 proteins or Beclin 1 and vimentin promoted by active Akt. (A) Immunoprecipitation of 14-3-3 proteins with endogenous Beclin 1 in HeLa cells with or without starvation for 2 hours. (B to C) Endogenous Beclin 1 S295 phosphorylation (B) and immunoprecipitation of endogenous 14-3-3 and vimentin with endogenous Beclin 1 (C) in Rat2 fibroblasts tranduced with control vector or myr-Akt1. (D) Immunoprecipitation of endogenous 14-3-3 and vimentin with Flag-Beclin 1 in Rat2 cells transduced with indicated Flag-Beclin 1 and Akt constructs. *, non-specific band. (E) Localization of Flag-Beclin 1 with endogenous vimentin in Rat2 cells transduced with the indicated vectors. WCL, whole cell lysates.
Fig. 4
Effects of vimentin on autophagy and Akt-mediated transformation. (A) GFP-LC3 dots in Rat2 cells transduced with indicated vimentin shRNA and transfected with GFP-LC3. Bars represent mean ± SEM of triplicate samples with >50 cells analyzed per sample. Similar results observed in 3 independent experiments. (B) Biochemical assessment of autophagy in Rat2 cells transduced with indicated vimentin shRNA and myr-Akt1 or control vector. (C) Soft agar colonies formed by Rat2 fibroblasts transduced with indicated vectors. Bars represent mean ± SEM of triplicate samples of ≥12 random images analyzed by ImageJ. (D) Speculative model for relations between Akt phosphorylation of Beclin 1; formation of a complex with Beclin 1, 14-3-3 proteins, and intermediate filament (IF) proteins; autophagy, and tumorigenesis. *P<0.05, **P<0.01, ***P<0.001; t test. In (A) and (C), all comparisons are with the first bar in graph.
Comment in
- Cell biology. Promoting tumorigenesis by suppressing autophagy.
Koren I, Kimchi A. Koren I, et al. Science. 2012 Nov 16;338(6109):889-90. doi: 10.1126/science.1230577. Science. 2012. PMID: 23161981 No abstract available.
Similar articles
- The XAF1 tumor suppressor induces autophagic cell death via upregulation of Beclin-1 and inhibition of Akt pathway.
Sun PH, Zhu LM, Qiao MM, Zhang YP, Jiang SH, Wu YL, Tu SP. Sun PH, et al. Cancer Lett. 2011 Nov 28;310(2):170-80. doi: 10.1016/j.canlet.2011.06.037. Epub 2011 Jul 5. Cancer Lett. 2011. PMID: 21788101 - Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation.
Pang XL, He G, Liu YB, Wang Y, Zhang B. Pang XL, et al. World J Gastroenterol. 2013 Mar 21;19(11):1736-48. doi: 10.3748/wjg.v19.i11.1736. World J Gastroenterol. 2013. PMID: 23555162 Free PMC article. - Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells.
Wang KF, Yang H, Jiang WQ, Li S, Cai YC. Wang KF, et al. Int J Mol Med. 2015 Dec;36(6):1556-62. doi: 10.3892/ijmm.2015.2378. Epub 2015 Oct 16. Int J Mol Med. 2015. PMID: 26499488 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - The Beclin 1 network regulates autophagy and apoptosis.
Kang R, Zeh HJ, Lotze MT, Tang D. Kang R, et al. Cell Death Differ. 2011 Apr;18(4):571-80. doi: 10.1038/cdd.2010.191. Epub 2011 Feb 11. Cell Death Differ. 2011. PMID: 21311563 Free PMC article. Review.
Cited by
- ATG16L1: A multifunctional susceptibility factor in Crohn disease.
Salem M, Ammitzboell M, Nys K, Seidelin JB, Nielsen OH. Salem M, et al. Autophagy. 2015 Apr 3;11(4):585-94. doi: 10.1080/15548627.2015.1017187. Autophagy. 2015. PMID: 25906181 Free PMC article. Review. - A functional outside-in signaling network of proteoglycans and matrix molecules regulating autophagy.
Neill T, Kapoor A, Xie C, Buraschi S, Iozzo RV. Neill T, et al. Matrix Biol. 2021 Jun;100-101:118-149. doi: 10.1016/j.matbio.2021.04.001. Epub 2021 Apr 7. Matrix Biol. 2021. PMID: 33838253 Free PMC article. - Cytoskeletal vimentin regulates cell size and autophagy through mTORC1 signaling.
Mohanasundaram P, Coelho-Rato LS, Modi MK, Urbanska M, Lautenschläger F, Cheng F, Eriksson JE. Mohanasundaram P, et al. PLoS Biol. 2022 Sep 13;20(9):e3001737. doi: 10.1371/journal.pbio.3001737. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36099296 Free PMC article. - Akt Is S-Palmitoylated: A New Layer of Regulation for Akt.
Blaustein M, Piegari E, Martínez Calejman C, Vila A, Amante A, Manese MV, Zeida A, Abrami L, Veggetti M, Guertin DA, van der Goot FG, Corvi MM, Colman-Lerner A. Blaustein M, et al. Front Cell Dev Biol. 2021 Feb 15;9:626404. doi: 10.3389/fcell.2021.626404. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33659252 Free PMC article. - Nrf2 but not autophagy inhibition is associated with the survival of wild-type epidermal growth factor receptor non-small cell lung cancer cells.
Zhou Y, Li Y, Ni HM, Ding WX, Zhong H. Zhou Y, et al. Toxicol Appl Pharmacol. 2016 Nov 1;310:140-149. doi: 10.1016/j.taap.2016.09.010. Epub 2016 Sep 14. Toxicol Appl Pharmacol. 2016. PMID: 27639429 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA084254/CA/NCI NIH HHS/United States
- K08 CA164047/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA129451/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- R01 CA071443/CA/NCI NIH HHS/United States
- R01 CA109618/CA/NCI NIH HHS/United States
- R01 CA84254-S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases