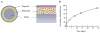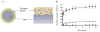Recent advances in drug eluting stents - PubMed (original) (raw)
Review
Recent advances in drug eluting stents
Amey S Puranik et al. Int J Pharm. 2013.
Abstract
One of the most common medical interventions to reopen an occluded vessel is the implantation of a coronary stent. While this method of treatment is effective initially, restenosis, or the re-narrowing of the artery frequently occurs largely due to neointimal hyperplasia of smooth muscle cells. Drug eluting stents were developed in order to provide local, site-specific, controlled release of drugs that can inhibit neointima formation. By implementing a controlled release delivery system it may be possible to control the time release of the pharmacological factors and thus be able to bypass some of the critical events associated with stent hyperplasia and prevent the need for subsequent intervention. However, since the advent of first-generation drug eluting stents, long-term adverse effects have raised concerns regarding their safety. These limitations in safety and efficacy have triggered considerable research in developing biodegradable stents and more potent drug delivery systems. In this review, we shed light on the current state-of-the-art in drug eluting stents, problems related to them and highlight some of the ongoing research in this area.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Fig. 1
Illustration of the mechanisms leading to (A) Atherosclerosis, (B) Restenosis due to angioplasty and (C) in-stent restenosis. (A) Dysfunction of endothelial cells triggers a response-to-injury mechanism increasing monocyte and lymphocyte adherence to the endothelium. Interaction of these cells with the endothelium leads to recruitment of more monocytes/macrophages, lymphocytes, platelets, and smooth muscle cells. Lipid accumulation produces foam cells, which along with lymphocytes and smooth muscle cells drives plaque/lesion formation. (B) Angioplasty causes intimal and medial tears which attract platelets and result in thrombi formation. Smooth muscle cells and collagen then synthesized as a result of the response-to-injury mechanism lead to vessel remodeling. (C) Stent implantation causes endothelial denudation and crushing of the plaque triggering an inflammatory response. Eventually, smooth muscle cell migration and proliferation leads to formation of neointima.
Fig. 1
Illustration of the mechanisms leading to (A) Atherosclerosis, (B) Restenosis due to angioplasty and (C) in-stent restenosis. (A) Dysfunction of endothelial cells triggers a response-to-injury mechanism increasing monocyte and lymphocyte adherence to the endothelium. Interaction of these cells with the endothelium leads to recruitment of more monocytes/macrophages, lymphocytes, platelets, and smooth muscle cells. Lipid accumulation produces foam cells, which along with lymphocytes and smooth muscle cells drives plaque/lesion formation. (B) Angioplasty causes intimal and medial tears which attract platelets and result in thrombi formation. Smooth muscle cells and collagen then synthesized as a result of the response-to-injury mechanism lead to vessel remodeling. (C) Stent implantation causes endothelial denudation and crushing of the plaque triggering an inflammatory response. Eventually, smooth muscle cell migration and proliferation leads to formation of neointima.
Fig. 1
Illustration of the mechanisms leading to (A) Atherosclerosis, (B) Restenosis due to angioplasty and (C) in-stent restenosis. (A) Dysfunction of endothelial cells triggers a response-to-injury mechanism increasing monocyte and lymphocyte adherence to the endothelium. Interaction of these cells with the endothelium leads to recruitment of more monocytes/macrophages, lymphocytes, platelets, and smooth muscle cells. Lipid accumulation produces foam cells, which along with lymphocytes and smooth muscle cells drives plaque/lesion formation. (B) Angioplasty causes intimal and medial tears which attract platelets and result in thrombi formation. Smooth muscle cells and collagen then synthesized as a result of the response-to-injury mechanism lead to vessel remodeling. (C) Stent implantation causes endothelial denudation and crushing of the plaque triggering an inflammatory response. Eventually, smooth muscle cell migration and proliferation leads to formation of neointima.
Fig. 2
Schematic of sirolimus eluting Cypher stent: (A) cross-sectional and side views (B) in vitro sirolimus release profile Adapted from Acharya and Park (2006) by permission.
Figure 3
Schematic of paclitaxel eluting Taxus stent: (A) cross-sectional and side views (B) in vitro paclitaxel release profile Adapted from Acharya and Park, 2006 by permission.
Similar articles
- Comparison between catheter-based delivery of paclitaxel after bare-metal stenting and drug-eluting stents in coronary artery disease patients at high risk for in-stent restenosis.
El-Mokadem M, El-Ramly M, Hassan A, Boshra H, AbdelWahab A. El-Mokadem M, et al. Cardiovasc Revasc Med. 2017 Dec;18(8):596-600. doi: 10.1016/j.carrev.2017.05.018. Epub 2017 May 31. Cardiovasc Revasc Med. 2017. PMID: 28625402 Clinical Trial. - Drug-eluting stents.
García-García HM, Vaina S, Tsuchida K, Serruys PW. García-García HM, et al. Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review. - INtimal hyPerplasia evAluated by oCT in de novo COROnary lesions treated by drug-eluting balloon and bare-metal stent (IN-PACT CORO): study protocol for a randomized controlled trial.
Burzotta F, Brancati MF, Trani C, Porto I, Tommasino A, De Maria G, Niccoli G, Leone AM, Coluccia V, Schiavoni G, Crea F. Burzotta F, et al. Trials. 2012 May 6;13:55. doi: 10.1186/1745-6215-13-55. Trials. 2012. PMID: 22559260 Free PMC article. Clinical Trial. - Two-year outcomes after first- or second-generation drug-eluting or bare-metal stent implantation in all-comer patients undergoing percutaneous coronary intervention: a pre-specified analysis from the PRODIGY study (PROlonging Dual Antiplatelet Treatment After Grading stent-induced Intimal hyperplasia studY).
Valgimigli M, Tebaldi M, Borghesi M, Vranckx P, Campo G, Tumscitz C, Cangiano E, Minarelli M, Scalone A, Cavazza C, Marchesini J, Parrinello G; PRODIGY Investigators. Valgimigli M, et al. JACC Cardiovasc Interv. 2014 Jan;7(1):20-8. doi: 10.1016/j.jcin.2013.09.008. Epub 2013 Dec 11. JACC Cardiovasc Interv. 2014. PMID: 24332420 Clinical Trial. - Paclitaxel-eluting stents: current clinical experience.
Grube E, Buellesfeld L. Grube E, et al. Am J Cardiovasc Drugs. 2004;4(6):355-60. doi: 10.2165/00129784-200404060-00003. Am J Cardiovasc Drugs. 2004. PMID: 15554720 Review.
Cited by
- Enzyme Prodrug Therapy Engineered into Electrospun Fibers with Embedded Liposomes for Controlled, Localized Synthesis of Therapeutics.
Chandrawati R, Olesen MTJ, Marini TCC, Bisra G, Guex AG, de Oliveira MG, Zelikin AN, Stevens MM. Chandrawati R, et al. Adv Healthc Mater. 2017 Sep;6(17):10.1002/adhm.201700385. doi: 10.1002/adhm.201700385. Epub 2017 Jul 12. Adv Healthc Mater. 2017. PMID: 28699219 Free PMC article. - A comprehensive study on venous endovascular management and stenting in deep veins occlusion and stenosis: A review study.
Salimi J, Chinisaz F, Yazdi SAM. Salimi J, et al. Surg Open Sci. 2024 Apr 16;19:131-140. doi: 10.1016/j.sopen.2024.04.001. eCollection 2024 Jun. Surg Open Sci. 2024. PMID: 38690401 Free PMC article. Review. - In vitro and in vivo evaluation of a dextran-graft-polybutylmethacrylate copolymer coated on CoCr metallic stent.
Delattre C, Velazquez D, Roques C, Pavon-Djavid G, Ollivier V, Lokajczyk A, Avramoglou T, Gueguen V, Louedec L, Caligiuri G, Jandrot-Perrus M, Boisson-Vidal C, Letourneur D, Meddahi-Pelle A. Delattre C, et al. Bioimpacts. 2019;9(1):25-36. doi: 10.15171/bi.2019.04. Epub 2018 Oct 2. Bioimpacts. 2019. PMID: 30788257 Free PMC article. - Clinical outcomes of ultrathin biodegradable polymer-coated sirolimus-eluting stents in an all-comer population: One-year results from the T-FLEX registry including high-risk subgroups.
Pothineni RB, Vijan V, Potdar A, Inamdar MK, Pathak A, Mantravadi SS, Ajmera P. Pothineni RB, et al. Anatol J Cardiol. 2021 Oct;25(10):706-715. doi: 10.5152/AnatolJCardiol.2021.78291. Anatol J Cardiol. 2021. PMID: 34622785 Free PMC article. - Mechanical properties of a biodegradable self-expandable polydioxanone monofilament stent: In vitro force relaxation and its clinical relevance.
Bezrouk A, Hosszu T, Hromadko L, Olmrova Zmrhalova Z, Kopecek M, Smutny M, Selke Krulichova I, Macak JM, Kremlacek J. Bezrouk A, et al. PLoS One. 2020 Jul 8;15(7):e0235842. doi: 10.1371/journal.pone.0235842. eCollection 2020. PLoS One. 2020. PMID: 32639989 Free PMC article.
References
- Acharya G, Park K. Mechanisms of controlled drug release from drug-eluting stents. Adv Drug Deliv Rev. 2006;58:387–401. - PubMed
- Ako J, Bonneau HN, Honda Y, Fitzgerald PJ. Design criteria for the ideal drug-eluting Stent. Am J Cardiol. 2007;100:3M–9M. - PubMed
- Babapulle MN, Eisenberg MJ. Coated stents for the prevention of restenosis: Part I. Circulation. 2002;106:2734–2740. - PubMed
- Banai S, Chorny M, Gertz SD, Fishbein I, Gao JC, Perez L, Lazarovichi G, Gazit A, Levitzki A, Golomb G. Locally delivered nanoencapsulated tyrphostin (AGL-2043) reduces neointima formation in balloon-injured rat carotid and stented porcine coronary arteries. Biomaterials. 2005;26:451–461. - PubMed
- Bartorelli AL, Trabattoni D, Fabbiocchi F, Montorsi P, de Martini S, Calligaris G, Teruzzi G, Galli S, Ravagnani P. Synergy of passive coating and targeted drug delivery: the tacrolimus-eluting Janus CarboStent. J Interv Cardiol. 2003;16:499–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical