Use the force: membrane tension as an organizer of cell shape and motility - PubMed (original) (raw)
Review
Use the force: membrane tension as an organizer of cell shape and motility
Alba Diz-Muñoz et al. Trends Cell Biol. 2013 Feb.
Abstract
Many cell phenomena that involve shape changes are affected by the intrinsic deformability of the plasma membrane (PM). Far from being a passive participant, the PM is now known to physically, as well as biochemically, influence cell processes ranging from vesicle trafficking to actin assembly. Here we review current understanding of how changes in PM tension regulate cell shape and movement, as well as how cells sense PM tension.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
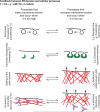
Figure 1. Feedback between plasma membrane tension and cellular processes
Examples of cellular processes that occur when PM tension is too high and that lead to its reduction (left) or that occur when PM tension is too low and lead to its increase (right) — vesicle trafficking, caveolae formation, actin polymerization and changes in myosin based. In brackets we comment on the parameters of Eq. 1 and 2 in Box 2 that are predicted to change in each of these processes.
Figure 2. Sensing membrane tension
PM in a resting cell (left) or following an increase in PM tension, as observed during cell protrusion or cell spreading (right). PM tension could be sensed by the opening of stretch activated ion channels (top), the dissociation of curvature-sensitive membrane-binding proteins (middle), or changes in the activity of membrane-to-cortex attachment proteins (bottom).
Similar articles
- Membrane shaping by actin and myosin during regulated exocytosis.
Papadopulos A. Papadopulos A. Mol Cell Neurosci. 2017 Oct;84:93-99. doi: 10.1016/j.mcn.2017.05.006. Epub 2017 May 20. Mol Cell Neurosci. 2017. PMID: 28536001 Review. - Reciprocal link between cell biomechanics and exocytosis.
Wang G, Galli T. Wang G, et al. Traffic. 2018 Oct;19(10):741-749. doi: 10.1111/tra.12584. Epub 2018 Jul 19. Traffic. 2018. PMID: 29943478 Review. - Caveolae: Mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation.
Del Pozo MA, Lolo FN, Echarri A. Del Pozo MA, et al. Curr Opin Cell Biol. 2021 Feb;68:113-123. doi: 10.1016/j.ceb.2020.10.008. Epub 2020 Nov 11. Curr Opin Cell Biol. 2021. PMID: 33188985 Review. - Membrane tension controls adhesion positioning at the leading edge of cells.
Pontes B, Monzo P, Gole L, Le Roux AL, Kosmalska AJ, Tam ZY, Luo W, Kan S, Viasnoff V, Roca-Cusachs P, Tucker-Kellogg L, Gauthier NC. Pontes B, et al. J Cell Biol. 2017 Sep 4;216(9):2959-2977. doi: 10.1083/jcb.201611117. Epub 2017 Jul 7. J Cell Biol. 2017. PMID: 28687667 Free PMC article. - Caveolae Mechanotransduction at the Interface between Cytoskeleton and Extracellular Matrix.
Sotodosos-Alonso L, Pulgarín-Alfaro M, Del Pozo MA. Sotodosos-Alonso L, et al. Cells. 2023 Mar 20;12(6):942. doi: 10.3390/cells12060942. Cells. 2023. PMID: 36980283 Free PMC article. Review.
Cited by
- Mechanical properties of external confinement modulate the rounding dynamics of cells.
Yang Y, Jiang H. Yang Y, et al. Biophys J. 2021 Jun 1;120(11):2306-2316. doi: 10.1016/j.bpj.2021.04.006. Epub 2021 Apr 20. Biophys J. 2021. PMID: 33864788 Free PMC article. - Membrane tension and peripheral protein density mediate membrane shape transitions.
Shi Z, Baumgart T. Shi Z, et al. Nat Commun. 2015 Jan 8;6:5974. doi: 10.1038/ncomms6974. Nat Commun. 2015. PMID: 25569184 Free PMC article. - HiViPore: a highly viable in-flow compression for a one-step cell mechanoporation in microfluidics to induce a free delivery of nano- macro-cargoes.
Maremonti MI, Panzetta V, Netti PA, Causa F. Maremonti MI, et al. J Nanobiotechnology. 2024 Jul 27;22(1):441. doi: 10.1186/s12951-024-02730-y. J Nanobiotechnology. 2024. PMID: 39068464 Free PMC article. - Membrane vectorial lipidomic features of coral host cells' plasma membrane and lipid profiles of their endosymbionts Cladocopium.
Sikorskaya TV, Ermolenko EV, Ginanova TT, Boroda AV, Efimova KV, Bogdanov M. Sikorskaya TV, et al. Commun Biol. 2024 Jul 18;7(1):878. doi: 10.1038/s42003-024-06578-8. Commun Biol. 2024. PMID: 39025984 Free PMC article. - Value of models for membrane budding.
Lee CT, Akamatsu M, Rangamani P. Lee CT, et al. Curr Opin Cell Biol. 2021 Aug;71:38-45. doi: 10.1016/j.ceb.2021.01.011. Epub 2021 Mar 8. Curr Opin Cell Biol. 2021. PMID: 33706232 Free PMC article. Review.
References
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nature Cell Biology. 2010;12:533–542. - PubMed
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282:F179–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources