Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease - PubMed (original) (raw)
Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease
Rongqing Chen et al. Cell Rep. 2012.
Abstract
Alzheimer's disease (AD) is the most common cause of dementia among older people. There are no effective medications currently available to prevent and treat AD and halt disease progression. Monoacylglycerol lipase (MAGL) is the primary enzyme metabolizing the endocannabinoid 2-arachidonoylglycerol in the brain. We show here that inactivation of MAGL robustly suppressed production and accumulation of β-amyloid (Aβ) associated with reduced expression of β-site amyloid precursor protein cleaving enzyme 1 (BACE1) in a mouse model of AD. MAGL inhibition also prevented neuroinflammation, decreased neurodegeneration, maintained integrity of hippocampal synaptic structure and function, and improved long-term synaptic plasticity, spatial learning, and memory in AD animals. Although the molecular mechanisms underlying the beneficial effects produced by MAGL inhibition remain to be determined, our results suggest that MAGL, which regulates endocannabinoid and prostaglandin signaling, contributes to pathogenesis and neuropathology of AD, and thus is a promising therapeutic target for the prevention and treatment of AD.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Statement of conflicts of interest
The authors state no conflict of interest.
Figures
Figure 1
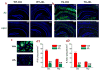
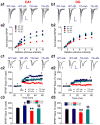
MAGL inhibition reduces production and deposition of Aβ in 5XFAD APP transgenic (TG) mice. a, b, Accumulation and deposition of total Aβ (all forms) and Aβ42 in 6 months old TG and their age-matched WT mice that received the vehicle and JZL184 (12 mg/kg, i.p.) three times per week starting at 4 months of age for 8 weeks. Total Aβ and Aβ42 were detected using immunohistochemistry with antibodies specific for all forms of Aβ (4G8, green) and for Aβ42 (green). Cell nuclei in the sections were stained with DAPI (Blue). Scale Bars: 400 μm. c, Magnification of Aβ plaques in vehicle- and JZL-treated TG mice. d, Number of total Aβ and Aβ42 in the cortex and hippocampus in vehicle- and JZL-treated TG mice. Data are means ±SEM from 5 to 6 mice per group. **P<0.01 compared with the vehicle control.
Figure 2
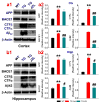
MAGL inhibitor JZL184 decreases expression of BACE1 and production of Aβ in TG mice. a, Immunoblot analysis of APP, BACE1, CTFβ/α, and Aβ42 in the cortex in 6 months old TG and their age-matched WT mice treated with the vehicle and JZL184 for 8 weeks. b, Immunoblot analysis of APP, BACE1, CTFβ/α, and Aβ42 in the hippocampus in vehicle- and JZL-treated TG mice. Data are means ±SEM from 4 to 6 mice per group. **P<0.01 compared with the WT vehicle control; §§P<0.01 compared with the TG vehicle control.
Figure 3
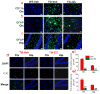
MAGL inhibition prevents neuroinflammation and reduces neurodegeneration in TG mice. a, Reactive microglial cells (CD11b/OX42, a microglial marker, green) are suppressed in 6 month old TG mice treated with JZL184 for 8 weeks. Scale bars: 200 μm. b, c, Activated astrocytes (GFAP, an astrocytic marker, green) in the cortex and hippocampus are reduced in TG mice that received JZL184 for 8 weeks. Scale bars: 100 μm. d, e, Degenerated neurons (FJC, a neurodegeneration marker, green, DAPI: red) are reduced in the cortex and hippocampus in TG mice treated with JZL184 for 8 (e1) and 16 (e2) weeks. **P<0.01 compared with the TG vehicle control (Data are averaged from 3 to 4 sections in three groups of mice, n=4 mice per group).
Figure 4
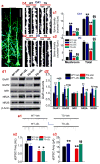
MAGL inactivation maintains integrity of hippocampal synaptic structure and function in TG mice. a, Two-photon image of GFP-expressing pyramidal neurons in the hippocampus from a transgenic mouse that expresses GFP in principal neurons. Scale bar: 50 μm. b, Segments of dendrites of pyramidal neurons in the CA1 region (b1) and of granule neurons in the dentate gyrus (DG, b2) in 6 months old WT-GFP- and TG-GFP-expressing animals that received vehicle or JZL184 for 8 weeks. Scale bars: 2 μm. c, Density of mushroom and total dendritic spines in CA1 pyramidal neurons (c1) and DG granule neurons (c2). The data represent mean values averaged from 43 to 56 images/group and 5 to 7 mice/group. **P<0.01 compared with the WT vehicle control; §§P<0.01 compared with the TG vehicle control. d, Immunoblot analysis of hippocampal expression of AMPA (GluR1 and GluR2) and NMDA (NR1, NR2A and NR2B) receptor subunits in 6 months old WT and TG mice treated with vehicle or JZL184 for 8 weeks. e, Spontaneous excitatory postsynaptic currents (sEPSCs) recorded in hippocampal CA1 pyramidal neurons in 6 months old WT and TG mice treated with vehicle or JZL184 for 8 weeks. Frequency and amplitude of sEPSCs were analyzed using the MiniAnalysis program. *P<0.05; **P<0.01 compared with the WT vehicle control, §P<0.05, §§P<0.01 compared with the TG vehicle control. 14 to 20 recordings were made in each group of animals (3 to 4 mice per group).
Figure 5
MAGL inhibition improves basal synaptic transmission and long-term synaptic plasticity in both WT and TG mice. a1, a2, Representative fEPSP waveforms recorded at hippocampal CA3-CA1 synapses and input-output function in 6 months old WT and TG injected with vehicle or JZl184 for 8 weeks (16 to 22 recordings/group and 5 to 8 mice/group). b1, b2, Representative fEPSP waveforms recorded at perforant path synapses and input-output function in 6 months old WT and TG injected with vehicle or JZl184 for 8 weeks (15 to 16 recordings/group and 5 to 6 mice/group). Stimulus intensity was normalized to the maximum intensity. Scale bar: 0.3 mV/10 msec. c1–c3, Representative fEPSP waveforms recorded at hippocampal CA3-CA1 synapses, LTP curves and mean values of fEPSP slope averaged from 56 to 60 min following theta-burst stimulation (TBS) in 6 months old WT and TG injected with vehicle or JZl184 for 8 weeks (10 to 14 recordings/group and 7 to 8 mice/group). d1–d3, Representative fEPSP waveforms recorded at perforant path synapses, LTP curves and mean values of fEPSP slope averaged from 56 to 60 min following TBS in 6 months old WT and TG injected with vehicle or JZl184 for 8 weeks (10 to 13 recordings/group and 5 to 8 mice/group). *P<0.05; **P<0.01 compared with the WT vehicle control; §P<0.05, §§P<0.01 compared with the TG vehicle control.
Figure 6
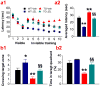
MAGL inhibition prevents spatial learning and memory deterioration in TG mice and enhances spatial learning and memory in WT mice. a, Spatial learning in the Morris water maze in 6-month-old WT and TG mice that received vehicle or JZL184 for 8 weeks. The test was conducted one week following the cessation of the treatment. The submerged platform was located in the center of one quadrant of the pool. Mice received visible platform training for 3 days followed by 7 days of invisible platform training. b, Averaged latency in 7 days of invisible training. Retention memory was determined using a probe trial test conducted 24 hrs after 7 days of training. During the probe test, the platform was removed from the pool. c, Number of times crossing the target zone, and d, Percentages of time stayed in the target quadrant. *P<0.05, **<0.01, compared with the WT vehicle control; §P<0.05, §§P<0.01 compared with the TG vehicle (n=9 to 12 mice/group).
Figure 7
MAGL inhibition-produced suppression of neuroinflammation is mediated neither by CB1R nor by CB2R in AD animals. 5XFAD transgenic mice were crossed with CB1 receptor knockout (CB1RKO) or CB2 receptor knockout mice (CB2RKO) to generate 5XFAD TG mice lacking CB1R or CB2R. a & b, Reactive astrocytes (GFAP, an astrocytic marker, green) are suppressed in 6 month old TG mice lacking CB1R or CB2R treated with JZL184 for 8 weeks. Scale bars: 50 μm.
Similar articles
- Alleviation of Neuropathology by Inhibition of Monoacylglycerol Lipase in APP Transgenic Mice Lacking CB2 Receptors.
Zhang J, Chen C. Zhang J, et al. Mol Neurobiol. 2018 Jun;55(6):4802-4810. doi: 10.1007/s12035-017-0689-x. Epub 2017 Jul 21. Mol Neurobiol. 2018. PMID: 28733897 Free PMC article. - Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer's disease.
Zhang J, Hu M, Teng Z, Tang YP, Chen C. Zhang J, et al. J Neurosci. 2014 Nov 5;34(45):14919-33. doi: 10.1523/JNEUROSCI.1165-14.2014. J Neurosci. 2014. PMID: 25378159 Free PMC article. - Inhibition of 2-Arachidonoylglycerol Metabolism Alleviates Neuropathology and Improves Cognitive Function in a Tau Mouse Model of Alzheimer's Disease.
Hashem J, Hu M, Zhang J, Gao F, Chen C. Hashem J, et al. Mol Neurobiol. 2021 Aug;58(8):4122-4133. doi: 10.1007/s12035-021-02400-2. Epub 2021 May 3. Mol Neurobiol. 2021. PMID: 33939165 Free PMC article. - Consequences of inhibiting amyloid precursor protein processing enzymes on synaptic function and plasticity.
Wang H, Megill A, He K, Kirkwood A, Lee HK. Wang H, et al. Neural Plast. 2012;2012:272374. doi: 10.1155/2012/272374. Epub 2012 Jun 26. Neural Plast. 2012. PMID: 22792491 Free PMC article. Review. - BACE1: the beta-secretase enzyme in Alzheimer's disease.
Vassar R. Vassar R. J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
- Endocannabinoid System Biomarkers in Alzheimer's Disease.
Ferreira PCL, Bellaver B, Povala G, Brum WS, Tissot C, Badji A, Sloan ME, Benedet AL, Rosa-Neto P, Ashton NJ, Pascoal TA, Leuzy A, Zimmer ER. Ferreira PCL, et al. Cannabis Cannabinoid Res. 2023 Feb;8(1):77-91. doi: 10.1089/can.2022.0151. Epub 2022 Nov 17. Cannabis Cannabinoid Res. 2023. PMID: 36394442 Free PMC article. - A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.
Devi L, Ohno M. Devi L, et al. Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article. - Overabundant endocannabinoids in neurons are detrimental to cognitive function.
Zhu D, Zhang J, Ma X, Hu M, Gao F, Hashem JB, Lyu J, Wei J, Cui Y, Qiu S, Chen C. Zhu D, et al. bioRxiv [Preprint]. 2024 Sep 17:2024.09.17.613513. doi: 10.1101/2024.09.17.613513. bioRxiv. 2024. PMID: 39345517 Free PMC article. Preprint. - Endocannabinoid signaling in brain diseases: Emerging relevance of glial cells.
Bernal-Chico A, Tepavcevic V, Manterola A, Utrilla C, Matute C, Mato S. Bernal-Chico A, et al. Glia. 2023 Jan;71(1):103-126. doi: 10.1002/glia.24172. Epub 2022 Mar 30. Glia. 2023. PMID: 35353392 Free PMC article. Review. - Inhibition of 2-arachidonoylglycerol degradation enhances glial immunity by single-cell transcriptomic analysis.
Zhu D, Zhang J, Hashem J, Gao F, Chen C. Zhu D, et al. J Neuroinflammation. 2023 Jan 30;20(1):17. doi: 10.1186/s12974-023-02701-4. J Neuroinflammation. 2023. PMID: 36717883 Free PMC article.
References
- Alger BE. Endocannabinoid signaling in neural plasticity. Curr Top Behav Neurosci. 2009;1:141–172. - PubMed
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. - PubMed
- Arevalo-Martin A, Garcia-Ovejero D, Molina-Holgado E. The endocannabinoid 2-arachidonoylglycerol reduces lesion expansion and white matter damage after spinal cord injury. Neurobiol Dis. 2010;38:304–312. - PubMed
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS076815/NS/NINDS NIH HHS/United States
- R01 NS054886/NS/NINDS NIH HHS/United States
- R21 AG039669/AG/NIA NIH HHS/United States
- R21AG039669/AG/NIA NIH HHS/United States
- R01NS054886/NS/NINDS NIH HHS/United States
- R01NS076815/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous