Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease - PubMed (original) (raw)
Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease
Nicolas F Berbari et al. Cytoskeleton (Hoboken). 2013 Jan.
Abstract
Disruption of the primary cilium is associated with a growing number of human diseases collectively termed ciliopathies. Ciliopathies present with a broad range of clinical features consistent with the near ubiquitous nature of the organelle and its role in diverse signaling pathways throughout development and adult homeostasis. The clinical features associated with cilia dysfunction can include such phenotypes as polycystic kidneys, skeletal abnormalities, blindness, anosmia, and obesity. Although the clinical relevance of the primary cilium is evident, the effects that cilia dysfunction has on the cell and how this contributes to disease remains poorly understood. Here, we show that loss of ciliogenesis genes such as Ift88 and Kif3a lead to increases in post-translational modifications on cytosolic microtubules. This effect was observed in cilia mutant kidney cells grown in vitro and in vivo in cystic kidneys. The hyper-acetylation of microtubules resulting from cilia loss is associated with both altered microtubule stability and increased α-tubulin acetyl-transferase activity. Intriguingly, the effect on microtubules was also evident in renal samples from patients with autosomal recessive polycystic kidneys. These findings indicate that altered microtubule post-translational modifications may influence some of the phenotypes observed in ciliopathies.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
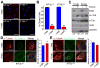
Figure 1. Loss of primary cilia is associated with increasedα-tubulin acetylation
(A) Immunofluorescence staining for the cilia marker Arl13b (red) in wildtype noninduced (−TAM) and tamoxifen-induced (+TAM) _Kif3a_flox/flox; CAAG-CreER (Kif3afl/fl) and _IFT88_flox/flox; CAAG-CreER (IFT88fl/fl) kidney epithelial cells. Scale bars 21 μm. (B) Quantification of cilia in control (−Tam) and mutant (+Tam) Kif3afl/fl and IFT88fl/fl cells. Bars show mean percent ciliated cells ± SEM, asterisks indicate a significant change of measurements compared with the control group (Student’s _t_-test; **P < 0.01). (C) Western blot analysis for α-tubulin acetylation, total α-tubulin, and actin using 10 μg and 20 μg of protein Kif3a mutant and control cells. Loading control is GAPDH. Numerical values at bottom are the densitometric ratio of acetylated α-tubulin to GAPDH normalized to the most intense band. (D, left panel) Immunofluorescence for α-tubulin (red) and acetylated-α-tubulin (green) in Kif3afl/fl conditional cells. Scale bar: 14 μm. (D, right panel) A graph measuring the fluorescence intensity of acetylated α-tubulin staining comparing control (−TAM) and Kif3a mutant (+TAM) cells. Bars show mean acetylated tubulin intensity per μm2 of each cell ± SEM, asterisks indicate a significant change of measurements compared with the control group (Student’s _t_-test; *P < 0.05). (E, left panel) Immunofluorescence forα-tubulin (α-tubulin, red) and poly-glutamylated tubulin (Glu. Tubulin, green) in Kif3afl/fl conditional mutant cells treated or nontreated with tamoxifen. Scale bar: 20μm. All nuclei stained with Hoechst in blue. (E, right panel) A graph measuring the fluorescence intensity of glutamylated α-tubulin staining comparing wildtype (−TAM) and cilia mutant (+TAM) cells. Bars show mean glutamylated tubulin intensity per μm2 of each cell ± SEM (n=102,68; Student’s _t_-test; P = 0.09)
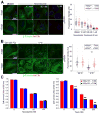
Figure 2. Microtubules in IFT/cilia mutants are more stable
(A) Immunofluorescence (left) analysis using cilia marker Arl13b (red) and β-tubulin (green) in ciliated (−TAM) and nonciliated (+TAM) Kif3a conditional cilia mutant cells treated with 10 nM, 100 nM and 1 μM nocodazole or DMSO. Quantification (right) of the β-tubulin fluorescence intensity. Dot plots show arbitrary fluorescence intensities of cells from background subtracted images. Lines indicate the mean and SEM. Asterisks indicate a significant change of measurements compared with the control (DMSO) group (Student’s _t_-test; **P < 0.01) (B) Immunofluorescence (left) for Arl13b (red) and for β-tubulin (green) in ciliated (−TAM) and nonciliated (+TAM) Kif3a conditional mutant cells after exposure to 4°C or 33°C for 90 minutes. Quantification (right) of the β-tubulin fluorescence intensity. Dot plots show arbitrary fluorescence intensities of cells from background subtracted images, lines indicate the mean and SEM. Asterisks indicate a significant change of measurements compared with their corresponding control (−TAM) group (Student’s _t_-test; **P < 0.01) (C) Quantification of cell survival in ciliated (−TAM) and nonciliated (+TAM) cells after nocodazole (left) and Taxol (right) treatment. Bars show mean fraction of surviving cells ± SEM. Asterisks indicate a significant change of measurements compared with the control group (Student’s _t_-test; **P < 0.01). Scale bars: 14 μm. Nuclei stained with Hoechst are blue.
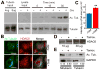
Figure 3. Increased microtubule acetylation activity in primary cilia mutant cells
(A) In vitro acetyl-transferase activity assay in cilia mutant and control cell extracts. Mec17 KO Tubulin substrate (Left panels, 4μg and 8μg of tubulin input alone) and 1μg of cell lysates from cilia mutants and controls (Middle panels, lysate inputs from Cre+ and Cre- cells) were immunoblotted for both acetylated α-tubulin (Ac. Tubulin) and total α-tubulin (Tubulin). Tubulin acetylation reactions from cilia mutant and control cells (Cre + and −) were run for 5, 15 and 30 minutes (Right panels). (B) Immunofluorescence for acetylated α-tubulin and histone deacetylase 6 in cilia mutant and control cells (+TAM and −TAM). Hoechst stained nuclei in blue. Scale bars are 14μm. (C) A graph measuring the fluorescence intensity of HDAC6 staining comparing control (−TAM) and Kif3a mutant (+TAM) cells. Bars represent mean HDAC6 intensity per μm2 of each cell ± SEM, asterisks indicate a significant change of measurements compared with the control group (Student’s _t_-test; **P < 0.01). (D) Western blot analysis for HDAC6 expression in cilia mutant (Tamox. +) and control cells (Tamox. −) with 10 μg and 30 μg of total protein. (E) Western blot analysis for α-tubulin acetylation in Kif3a mutant and control cells treated with tubacin or niltubacin control. Loading control is GAPDH. Numerical values at bottom are the normalized densitometric ratio of acetylated α-tubulin to GAPDH
Figure 4. Increasedα-tubulin acetylation in cilia mutant mouse tissue and human ARPKD cystic kidney disease patients
(A) Immunofluorescence for anti-γ-tubulin (purple), acetylated α-tubulin (green), and basement membrane marker entactin (red) in CAGG-CREER; IFT88fl/fl wildtype (Cre−) and cystic mutants (Cre+) kidneys. Scale bar 25 μm. (B) Immunofluorescence analysis of the total microtubule network stained withβ-tubulin (green) in cystic (Cre+) and non-cystic (Cre−) kidneys. All scale bars 15 μm. Hoechst stained nuclei in blue. (C) Western blot analysis of ciliated (Cre−) and nonciliated (Cre+) kidneys probed for IFT88, acetylated α-tubulin, α-tubulin, actin and GAPDH. Numerical values at bottom are densitometric ratio of acetylated α-tubulin to GAPDH normalized to the most intense band. (D) Western blot analysis of control Control (CTRL) and ARPKD human samples probed for acetylated α-tubulin, α-tubulin and total histone, numerical values at bottom are normalized densitometric ratio of acetylated α-tubulin to histone.
Similar articles
- Mks6 mutations reveal tissue- and cell type-specific roles for the cilia transition zone.
Lewis WR, Bales KL, Revell DZ, Croyle MJ, Engle SE, Song CJ, Malarkey EB, Uytingco CR, Shan D, Antonellis PJ, Nagy TR, Kesterson RA, Mrug MM, Martens JR, Berbari NF, Gross AK, Yoder BK. Lewis WR, et al. FASEB J. 2019 Jan;33(1):1440-1455. doi: 10.1096/fj.201801149R. Epub 2018 Aug 22. FASEB J. 2019. PMID: 30133325 Free PMC article. - Phosphatase inhibitor 2 promotes acetylation of tubulin in the primary cilium of human retinal epithelial cells.
Wang W, Brautigan DL. Wang W, et al. BMC Cell Biol. 2008 Nov 26;9:62. doi: 10.1186/1471-2121-9-62. BMC Cell Biol. 2008. PMID: 19036150 Free PMC article. - Ciliopathies and the Kidney: A Review.
McConnachie DJ, Stow JL, Mallett AJ. McConnachie DJ, et al. Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review. - The cilia protein IFT88 is required for spindle orientation in mitosis.
Delaval B, Bright A, Lawson ND, Doxsey S. Delaval B, et al. Nat Cell Biol. 2011 Apr;13(4):461-8. doi: 10.1038/ncb2202. Epub 2011 Mar 27. Nat Cell Biol. 2011. PMID: 21441926 Free PMC article. - Role of tubulin acetylation in cellular functions and diseases.
Nekooki-Machida Y, Hagiwara H. Nekooki-Machida Y, et al. Med Mol Morphol. 2020 Dec;53(4):191-197. doi: 10.1007/s00795-020-00260-8. Epub 2020 Jul 6. Med Mol Morphol. 2020. PMID: 32632910 Review.
Cited by
- Mks6 mutations reveal tissue- and cell type-specific roles for the cilia transition zone.
Lewis WR, Bales KL, Revell DZ, Croyle MJ, Engle SE, Song CJ, Malarkey EB, Uytingco CR, Shan D, Antonellis PJ, Nagy TR, Kesterson RA, Mrug MM, Martens JR, Berbari NF, Gross AK, Yoder BK. Lewis WR, et al. FASEB J. 2019 Jan;33(1):1440-1455. doi: 10.1096/fj.201801149R. Epub 2018 Aug 22. FASEB J. 2019. PMID: 30133325 Free PMC article. - Integrin-β1 is required for the renal cystogenesis caused by ciliary defects.
Yoo M, Barisoni LMC, Lee K, Gusella GL. Yoo M, et al. Am J Physiol Renal Physiol. 2020 May 1;318(5):F1306-F1312. doi: 10.1152/ajprenal.00070.2020. Epub 2020 Apr 20. Am J Physiol Renal Physiol. 2020. PMID: 32308017 Free PMC article. - Renal Ciliopathies: Sorting Out Therapeutic Approaches for Nephronophthisis.
Stokman MF, Saunier S, Benmerah A. Stokman MF, et al. Front Cell Dev Biol. 2021 May 13;9:653138. doi: 10.3389/fcell.2021.653138. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34055783 Free PMC article. Review. - A Cilia Independent Role of Ift88/Polaris during Cell Migration.
Boehlke C, Janusch H, Hamann C, Powelske C, Mergen M, Herbst H, Kotsis F, Nitschke R, Kuehn EW. Boehlke C, et al. PLoS One. 2015 Oct 14;10(10):e0140378. doi: 10.1371/journal.pone.0140378. eCollection 2015. PLoS One. 2015. PMID: 26465598 Free PMC article. - Destabilization of the IFT-B cilia core complex due to mutations in IFT81 causes a Spectrum of Short-Rib Polydactyly Syndrome.
Duran I, Taylor SP, Zhang W, Martin J, Forlenza KN, Spiro RP, Nickerson DA, Bamshad M, Cohn DH, Krakow D. Duran I, et al. Sci Rep. 2016 Sep 26;6:34232. doi: 10.1038/srep34232. Sci Rep. 2016. PMID: 27666822 Free PMC article.
References
- Bell PB, Jr, Safiejko-Mroczka B. Improved methods for preserving macromolecular structures and visualizing them by fluorescence and scanning electron microscopy. Scanning Microsc. 1995;9(3):843–57. discussion 858–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK075996/DK/NIDDK NIH HHS/United States
- 1F32DK088404/DK/NIDDK NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- R01 DK075996/DK/NIDDK NIH HHS/United States
- F32 DK088404/DK/NIDDK NIH HHS/United States
- R01 DK065655/DK/NIDDK NIH HHS/United States
- R56 DK075996/DK/NIDDK NIH HHS/United States
- GM089912/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases