Disulfide bond formation in the mammalian endoplasmic reticulum - PubMed (original) (raw)
Review
Disulfide bond formation in the mammalian endoplasmic reticulum
Neil J Bulleid. Cold Spring Harb Perspect Biol. 2012.
Abstract
The formation of disulfide bonds between cysteine residues occurs during the folding of many proteins that enter the secretory pathway. As the polypeptide chain collapses, cysteines brought into proximity can form covalent linkages during a process catalyzed by members of the protein disulfide isomerase family. There are multiple pathways in mammalian cells to ensure disulfides are introduced into proteins. Common requirements for this process include a disulfide exchange protein and a protein oxidase capable of forming disulfides de novo. In addition, any incorrect disulfides formed during the normal folding pathway are removed in a process involving disulfide exchange. The pathway for the reduction of disulfides remains poorly characterized. This work will cover the current knowledge in the field and discuss areas for future investigation.
Figures
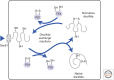
Figure 1.
PDI family of enzymes catalyzes disulfide exchange reactions in the endoplasmic reticulum. Nascent polypeptide chains are cotranslationally translocated across the ER membrane whereupon cysteines in close proximity can form disulfides. The reaction is catalyzed by members of the PDI family (depicted as PDI) by a disulfide exchange reaction resulting in the reduction of the PDI active site. If nonnative disulfides are formed these can be reduced by the reverse disulfide exchange reaction, resulting in the oxidation of the PDI active site.
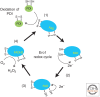
Figure 2.
Electron flow following the oxidation of PDI by Ero1. Ero1 accepts electrons from PDI (1) resulting in the oxidation of PDI and the reduction of the shuttle disulfide within Ero1. An internal disulfide exchange reaction then occurs within Ero1 (2) to transfer electrons to cysteines close to the bound FAD. FAD accepts electrons from the disulfide that is formed (3) and in the process is reduced to form FADH2. Oxygen is the ultimate electron acceptor (4) becoming reduced to liberate hydrogen peroxide.
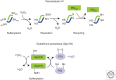
Figure 3.
Alternative pathways for PDI oxidation involving PrxIV or Gpx7/8. For both pathways the initial reaction is the oxidation of the active site cysteine to form sulfenylated cysteine. This residue is resolved by a second cysteine within an adjacent subunit in PrxIV or within the PDI active site for Gpx7/8. Reduced PDI recycles PrxIV to regenerate a free thiol at the active site of PrxIV and in the process becomes oxidized. Any mixed disulfide formed between PDI and Gpx7/8 will be rapidly resolved by the second cysteine in the PDI active site to form oxidized PDI. The cysteine residues marked “res” and “per” in PrxIV refer to the resolving and peroxidatic cysteines, respectively.
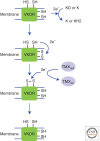
Figure 4.
VKOR can also oxidize members of the PDI family. VKOR is a membrane protein that contains two cysteines within a transmembrane helix. These can form a disulfide following the donation of electrons to either vitamin K epoxide (KO) or vitamin K (K), generating vitamin K hydroquinone (KH2). The resulting disulfide can then exchange with two cysteines present within the luminal domain of VKOR. The resulting disulfide can be reduced by the PDI family members TMX1, TMX4, or ERp18, resulting in the oxidation of these proteins.
Similar articles
- Multiple ways to make disulfides.
Bulleid NJ, Ellgaard L. Bulleid NJ, et al. Trends Biochem Sci. 2011 Sep;36(9):485-92. doi: 10.1016/j.tibs.2011.05.004. Epub 2011 Jul 19. Trends Biochem Sci. 2011. PMID: 21778060 Review. - Forming disulfides in the endoplasmic reticulum.
Oka OB, Bulleid NJ. Oka OB, et al. Biochim Biophys Acta. 2013 Nov;1833(11):2425-9. doi: 10.1016/j.bbamcr.2013.02.007. Epub 2013 Feb 20. Biochim Biophys Acta. 2013. PMID: 23434683 Review. - Pathways for protein disulphide bond formation.
Frand AR, Cuozzo JW, Kaiser CA. Frand AR, et al. Trends Cell Biol. 2000 May;10(5):203-10. doi: 10.1016/s0962-8924(00)01745-1. Trends Cell Biol. 2000. PMID: 10754564 Review. - Pathways of disulfide bond formation in Escherichia coli.
Messens J, Collet JF. Messens J, et al. Int J Biochem Cell Biol. 2006;38(7):1050-62. doi: 10.1016/j.biocel.2005.12.011. Epub 2006 Jan 11. Int J Biochem Cell Biol. 2006. PMID: 16446111 Review. - Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum.
Benham AM, van Lith M, Sitia R, Braakman I. Benham AM, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20110403. doi: 10.1098/rstb.2011.0403. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530257 Free PMC article.
Cited by
- On-column disulfide bond formation of monoclonal antibodies during Protein A chromatography eliminates low molecular weight species and rescues reduced antibodies.
Tan Z, Ehamparanathan V, Ren T, Tang P, Hoffman L, Kuang J, Liu P, Huang C, Du C, Tao L, Chemmalil L, Lewandowski A, Ghose S, Li ZJ, Liu S. Tan Z, et al. MAbs. 2020 Jan-Dec;12(1):1829333. doi: 10.1080/19420862.2020.1829333. MAbs. 2020. PMID: 33016217 Free PMC article. - Thiol-disulfide exchange between the PDI family of oxidoreductases negates the requirement for an oxidase or reductase for each enzyme.
Oka OB, Yeoh HY, Bulleid NJ. Oka OB, et al. Biochem J. 2015 Jul 15;469(2):279-88. doi: 10.1042/BJ20141423. Epub 2015 May 19. Biochem J. 2015. PMID: 25989104 Free PMC article. - A quantitative pipeline to assess secretion of human leptin coding variants reveals mechanisms underlying leptin deficiencies.
Baird HJM, Shun-Shion AS, Mendes de Oliveira E, Stalder D, Liang L, Eden J, Chambers JE, Farooqi IS, Gershlick DC, Fazakerley DJ. Baird HJM, et al. J Biol Chem. 2024 Aug;300(8):107562. doi: 10.1016/j.jbc.2024.107562. Epub 2024 Jul 19. J Biol Chem. 2024. PMID: 39002670 Free PMC article. - Pharmacognostic and pharmacological evaluation of Eulaliopsis binata plant extracts by measuring in vitro/in vivo safety profile and anti-microbial potential.
Kumar V, Sharma AK, Rajput SK, Pal M, Dhiman N. Kumar V, et al. Toxicol Res (Camb). 2018 Mar 12;7(3):454-464. doi: 10.1039/c8tx00017d. eCollection 2018 May 8. Toxicol Res (Camb). 2018. PMID: 30090595 Free PMC article. - Disulfide Bond Engineering of an Endoglucanase from Penicillium verruculosum to Improve Its Thermostability.
Bashirova A, Pramanik S, Volkov P, Rozhkova A, Nemashkalov V, Zorov I, Gusakov A, Sinitsyn A, Schwaneberg U, Davari MD. Bashirova A, et al. Int J Mol Sci. 2019 Mar 30;20(7):1602. doi: 10.3390/ijms20071602. Int J Mol Sci. 2019. PMID: 30935060 Free PMC article.
References
- Alberti A, Karamessinis P, Peroulis M, Kypreou K, Kavvadas P, Pagakis S, Politis PK, Charonis A 2009. ERp46 is reduced by high glucose and regulates insulin content in pancreatic β-cells. Am J Physiol Endocrinol Metab 297: E812–E821 - PubMed
- Arner ES 2009. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim Biophys Acta 1790: 495–526 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials