STING manifests self DNA-dependent inflammatory disease - PubMed (original) (raw)
STING manifests self DNA-dependent inflammatory disease
Jeonghyun Ahn et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Inflammatory autoimmune diseases such as systemic lupus erythematosus (SLE) and polyarthritis are characterized by chronic cytokine overproduction, suggesting that the stimulation of host innate immune responses, speculatively by persistent infection or self nucleic acids, plays a role in the manifestation of these disorders. Mice lacking DNase II die during embryonic development through comparable inflammatory disease because phagocytosed DNA from apoptotic cells cannot be adequately digested and intracellular host DNA sensor pathways are engaged, resulting in the production of a variety of cytokines including type I IFN. The cellular sensor pathway(s) responsible for triggering DNA-mediated inflammation aggravated autoimmune disease remains to be determined. However, we report here that Stimulator of IFN Genes (STING) is responsible for inflammation-related embryonic death in DNase II defective mice initiated by self DNA. DNase II-dependent embryonic lethality was rescued by loss of STING function, and polyarthritis completely prevented because cytosolic DNA failed to robustly trigger cytokine production through STING-controlled signaling pathways. Our data provides significant molecular insight into the causes of DNA-mediated inflammatory disorders and affords a target that could plausibly be therapeutically controlled to help prevent such diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
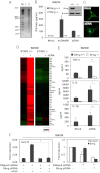
Role of STING in facilitating cytokines production by apoptotic DNA. (A) DNA from thymocytes treated with or without Dex (10 μM, 5 h). (B) STING expression as analyzed by immunoblot and IFNβ ELISA of Sting_+/+_ and Sting_−/−_ BMDM treated with dsDNA90 or aDNA. ND, nondetectable. (C) Confocal analysis of BMDMs transfected with aDNA and immunostained with anti-STING antibody. (Magnification: 1260×.) (D) Microarray of Sting_+/+_ or Sting_−/−_ BMDM transfected with aDNA (6 h). (E) ELISA of IL-β, IL-6, and TNFα in BMDMs treated with or without aDNA. (F) Cxcl10 mRNA level levels in BMDMs treated with STING and/or DNase II siRNA after exposure for 6 h to apoptotic thymocytes (aCell). Cxcl10 mRNA level was normalized to the expression of GAPDH (Left). *P < 0.05, **P < 0.005 by Student’s t test.
Fig. 2.
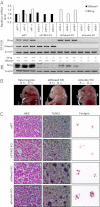
STING-dependent gene regulation in E17 Sting−/− DNase II−/−embryos. (A) STING and DNase II expression by qPCR and Western blot from E17 fetal liver wild-type (eWT), Sting−/− DNase II+/+(eStingKO), Sting+/− DNase II−/− (eDNaseIIKO), and Sting−/− DNase II−/− (eDoubleKO) embryos. (B) Macroscopic view of E17 Sting+/− DNase II+/−(Left), eDNaseIIKO (Center), and eDoubleKO (Right) embryos. (C) H&E and TUNEL stains from mice livers same as A. Feulgen stain of BMDM after incubation with thymocytes (+) Dex. (Magnification: 200×.)
Fig. 3.
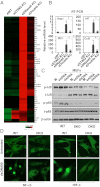
STING-dependent gene regulation in E17 Sting_−/−_ DNase II_−/−_ embryos. (A) Gene array analysis of fetal liver RNA isolated from E17 mice same as Fig. 2_A_. Data are from the mean of three independent embryos. (B) qPCR analysis of Ifnβ1, Irf7, Ifit3, and Ccl5 mRNA level from RNA same as (A). Error bars indicate SD. *P < 0.05, ***P < 0.0005 by Student’s t test. (C) Immunoblot analysis to determine the levels of p-Iκb and p-p65 in MEFs from Sting_+/+DNase II+/+_ (WT), Sting_−/−_ DNase II_+/+_ (SKO), and Sting_−/−_ DNase II_−/−_ (DKO) treated with dsDNA90 and ssDNA90 for 3 h. M, mock. (D) Confocal analysis of MEFs isolated from WT and E17 DKO embryos transfected with dsDNA90 for 3 h and stained with anti–NF-κB and IRF-3 antibody. (Magnification: 1260×.)
Fig. 4.
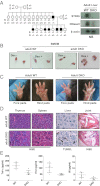
Generation and characterization of Sting_−/−_ DNase II_−/−_ adult mice. (A) Mendelian analysis of the progeny DKO_-deficient mice. Northern blotting with Sting and DNaseII cDNA as probe from liver of 8-wk-old Sting+/+DNase II+/+_ (WT), and DKO mice. (B) Feulgen stain of peritoneal macrophages stained with Schiff reagent after incubation with thymocytes treated with or without Dex (10 μM, 5 h). (C) Macroscopic view of the normal fore and hind paws of DKO at the age of 12 mo. (D) H&E stain of joint sections from 6-mo-old WT and DKO mice. B, bone; C, cartilage; S; synovial. (Magnification: 200×.) (E) TNF-α, IL1-β, and IL-6 levels in serum from 8-mo-old DKO was measured by ELISA. Wild-type mice are shown as control for DKO. Data are shown with mean of five independent mice. Error bars indicate SD.
Similar articles
- TBK1 recruitment to STING mediates autoinflammatory arthritis caused by defective DNA clearance.
Li T, Yum S, Li M, Chen X, Zuo X, Chen ZJ. Li T, et al. J Exp Med. 2022 Jan 3;219(1):e20211539. doi: 10.1084/jem.20211539. Epub 2021 Dec 13. J Exp Med. 2022. PMID: 34901991 Free PMC article. - Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease.
Ahn J, Barber GN. Ahn J, et al. Curr Opin Immunol. 2014 Dec;31:121-6. doi: 10.1016/j.coi.2014.10.009. Epub 2014 Nov 4. Curr Opin Immunol. 2014. PMID: 25459004 Review. - Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice.
Baum R, Sharma S, Carpenter S, Li QZ, Busto P, Fitzgerald KA, Marshak-Rothstein A, Gravallese EM. Baum R, et al. J Immunol. 2015 Feb 1;194(3):873-7. doi: 10.4049/jimmunol.1402573. Epub 2014 Dec 29. J Immunol. 2015. PMID: 25548216 Free PMC article. - STING Contributes to Abnormal Bone Formation Induced by Deficiency of DNase II in Mice.
Baum R, Sharma S, Organ JM, Jakobs C, Hornung V, Burr DB, Marshak-Rothstein A, Fitzgerald KA, Gravallese EM. Baum R, et al. Arthritis Rheumatol. 2017 Feb;69(2):460-471. doi: 10.1002/art.39863. Arthritis Rheumatol. 2017. PMID: 27740718 Free PMC article. - Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses.
Barber GN. Barber GN. Curr Opin Immunol. 2011 Feb;23(1):10-20. doi: 10.1016/j.coi.2010.12.015. Epub 2011 Jan 14. Curr Opin Immunol. 2011. PMID: 21239155 Free PMC article. Review.
Cited by
- Diverse roles of STING-dependent signaling on the development of cancer.
Ahn J, Konno H, Barber GN. Ahn J, et al. Oncogene. 2015 Oct 8;34(41):5302-8. doi: 10.1038/onc.2014.457. Epub 2015 Feb 2. Oncogene. 2015. PMID: 25639870 Free PMC article. - The kallikrein-kinin system in experimental Chagas disease: a paradigm to investigate the impact of inflammatory edema on GPCR-mediated pathways of host cell invasion by Trypanosoma cruzi.
Scharfstein J, Andrade D, Svensjö E, Oliveira AC, Nascimento CR. Scharfstein J, et al. Front Immunol. 2013 Jan 25;3:396. doi: 10.3389/fimmu.2012.00396. eCollection 2012. Front Immunol. 2013. PMID: 23355836 Free PMC article. - Role and Mechanism of cGAS-STING Pathway in Cardiovascular System.
Yu X, Pan S. Yu X, et al. Rev Cardiovasc Med. 2024 Apr 7;25(4):135. doi: 10.31083/j.rcm2504135. eCollection 2024 Apr. Rev Cardiovasc Med. 2024. PMID: 39076568 Free PMC article. Review. - Immune Sensing Mechanisms that Discriminate Self from Altered Self and Foreign Nucleic Acids.
Bartok E, Hartmann G. Bartok E, et al. Immunity. 2020 Jul 14;53(1):54-77. doi: 10.1016/j.immuni.2020.06.014. Immunity. 2020. PMID: 32668228 Free PMC article. Review. - Activation of cGAS/STING pathway upon paramyxovirus infection.
Iampietro M, Dumont C, Mathieu C, Spanier J, Robert J, Charpenay A, Dupichaud S, Dhondt KP, Aurine N, Pelissier R, Ferren M, Mély S, Gerlier D, Kalinke U, Horvat B. Iampietro M, et al. iScience. 2021 May 7;24(6):102519. doi: 10.1016/j.isci.2021.102519. eCollection 2021 Jun 25. iScience. 2021. PMID: 34142033 Free PMC article.
References
- Nagata S. Apoptosis and autoimmune diseases. Ann N Y Acad Sci. 2010;1209:10–16. - PubMed
- Helmick CG, et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. - PubMed
- Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. - PubMed
- Kawane K, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292(5521):1546–1549. - PubMed
- Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6(1):49–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials