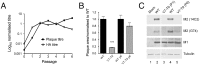Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain - PubMed (original) (raw)
doi: 10.1371/journal.ppat.1002998. Epub 2012 Nov 1.
Edward C Hutchinson, Brett W Jagger, Amanda D Stuart, Zi H Kang, Nicole Robb, Louis M Schwartzman, John C Kash, Ervin Fodor, Andrew E Firth, Julia R Gog, Jeffery K Taubenberger, Paul Digard
Affiliations
- PMID: 23133386
- PMCID: PMC3486900
- DOI: 10.1371/journal.ppat.1002998
Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain
Helen M Wise et al. PLoS Pathog. 2012.
Abstract
Segment 7 of influenza A virus produces up to four mRNAs. Unspliced transcripts encode M1, spliced mRNA2 encodes the M2 ion channel, while protein products from spliced mRNAs 3 and 4 have not previously been identified. The M2 protein plays important roles in virus entry and assembly, and is a target for antiviral drugs and vaccination. Surprisingly, M2 is not essential for virus replication in a laboratory setting, although its loss attenuates the virus. To better understand how IAV might replicate without M2, we studied the reversion mechanism of an M2-null virus. Serial passage of a virus lacking the mRNA2 splice donor site identified a single nucleotide pseudoreverting mutation, which restored growth in cell culture and virulence in mice by upregulating mRNA4 synthesis rather than by reinstating mRNA2 production. We show that mRNA4 encodes a novel M2-related protein (designated M42) with an antigenically distinct ectodomain that can functionally replace M2 despite showing clear differences in intracellular localisation, being largely retained in the Golgi compartment. We also show that the expression of two distinct ion channel proteins is not unique to laboratory-adapted viruses but, most notably, was also a feature of the 1983 North American outbreak of H5N2 highly pathogenic avian influenza virus. In identifying a 14th influenza A polypeptide, our data reinforce the unexpectedly high coding capacity of the viral genome and have implications for virus evolution, as well as for understanding the role of M2 in the virus life cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
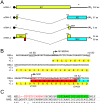
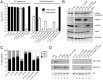
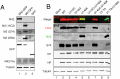
Figure 1. Segment 7 transcript and ORF structure.
(A). Diagrammatic summary of mRNA splice variants. The nucleotide coordinates of SD and SA sites are shown. Potential ORFs are colour coded (yellow, M1; blue, M2; red, unique sequence of M42) and total sizes (codons/aa) are given on the right. The arrowhead at top right indicates the binding site of the oligonucleotide used to detect the various mRNA species by radioactive primer extension reactions. (B) Nucleotide sequence (shown as cDNA) and predicted ORFs (colour coded as in (A)) of the 5′-end of PR8 mRNA4. Unused SD sequences and the splice junction (SJ) sequence are underlined. Nucleotides mutated to remove the M42 AUG codon (U115C) or abolish mRNA4 synthesis (G145A) are shown in red. (C) Alignment of the predicted N-terminal sequences of M2 and M42. The range of residues implicated in recognition of M2 by the 14C2 antibody are indicated in red –, . The transmembrane domain of M2 is shaded in green.
Figure 2. Pseudoreversion of an M2-null virus.
(A). Plaque and HA titre plotted before (passage 0) and after each step of 6 serial passage experiments as a fraction of the corresponding mean values obtained from two independent stocks of WT virus passaged in parallel. (B) Average plaque size in MDCK cells of the indicated viruses before and after (P6) serial passage. Values are the mean + SEM of 15–92 plaques normalized to the average WT value from each experiment. *** = p<0.001 compared to WT. (C). Segment 7 polypeptide expression. Lysates from cells infected with the indicated viruses were analysed by SDS-PAGE and western blotting as labeled.
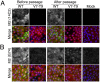
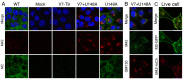
Figure 3. Immunofluorescent analysis of M2 expression.
MDCK cells were infected with the indicated viruses at an MOI of 10, fixed at 8h p.i., permeabilised and stained with (A) anti M2 14C2 or (B) G74 (in green, as labeled) and (as counterstains) with anti-NP (red) and DAPI (blue) before imaging by confocal microscopy. Extended focus projections of a series of optical sections through the depth of the cells are shown, either as merged 3-colour images or (in grey scale), the green channel alone.
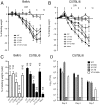
Figure 4. Genetic and biochemical evidence for pseudoreversion through upregulation of mRNA4.
(A) Endpoint titres after multicycle replication in MDCK cells of viruses with the indicated mutations to segment 7. Values are plotted are the mean + SEM of between 2 and 18 independent rescues. NR; not rescuable in 2 or more attempts. Viruses were also visually classified into normal (black bar) and small (white bar) plaque phenotypes. (B, C) Segment 7 mRNA accumulation. Total RNA isolated from cells infected with the indicated viruses at 6 h p.i. was analysed by RT-primer extension and urea-PAGE using primers specific for segment 7 mRNAs, vRNA or (as a loading control), cellular 5S rRNA. (C) The amounts of mRNAs 1–4 were quantified by phosphorimager and plotted as the mean ± SD of 3 experiments (D) Segment 7 polypeptide accumulation was monitored by western blot analysis of lysates from 293T cells transfected with reverse genetics plasmids for the indicated viruses at 72 h post transfection with the indicated antisera.
Figure 5. Direct detection of the M42 polypeptide.
(A) Validation of anti-M42 serum. Lysates from cells transfected with the indicated GFP polypeptides were analysed by SDS-PAGE and western blotting as labeled. (B) Detection of M42 from virus-infected cells. Lysates from cells infected with the indicated viruses at 10 h p.i. were analysed by SDS-PAGE and western blotting as labeled. The same membrane was probed with mouse anti-M2 14C2 and rabbit anti-M42 using different colour secondary antisera; individual grey scale and colour merged images are shown.
Figure 6. Intracellular localization of M42.
(A,B) MDCK cells were infected with the indicated viruses at an MOI of 3, fixed and permeabilised at 10 h p.i. and stained with DAPI (blue), anti-M42 and (A) anti-M2 14C2 or (B) anti-GM130. Single optical sections are shown, either as single channels or merged overlays as labeled. (C) A549 cells were transfected with M42-mCherry and M2-GFP plasmids and imaged at 37°C without fixation 16 h later. Single optical slices are shown. See also corresponding videos S1, S2, S3.
Figure 7. Pathogenicity of segment 7 mutant viruses in mice.
Mice were inoculated with 100 PFU of the indicated viruses and (A, B) weight loss measured on a daily basis. Mice were euthanised if their body weights fell below 25% of the starting value. (A) BALB/c mice were used. Data plotted are the mean and SEM values from two independent experiments each using 5 mice/group. (B, D) C57BL/6 mice were used. Data plotted are the mean and SEM values from a single experiment with 5 mice/group. (C) Mean and SEM nadir weights from (A, B) are plotted. Numbers over the bars indicate the number of mice that survived/total group size. (D) Lung titres (mean and SEM) from mice sacrificed at the indicated days. Three mice on days 2 and 7 and four mice on day 4 postinfection were used. Dashed line indicates the lower limit of detection.
Figure 8. Expression and functional significance of mRNA4 in other strains of IAV.
(A, B) Total RNA from cells infected with the indicated viruses at (A) an MOI of 5 and harvested at 6 h p.i. or (B) an MOI of ∼1 and harvested at 9 h p.i. was analysed by RT-primer extension for cellular 5S rRNA and virus-derived RNA species as labeled. (C) Endpoint titres after multicycle replication in MDCK cells of 7+1 reassortant PR8 viruses (PR8 MPenn) with the indicated mutations to Penn segment 7. Values are plotted are the mean + SEM of 3–14 independent rescues. NR; not rescued in 3 attempts. Viruses were also visually classified into normal (black bar) and small (open bar) plaque phenotypes.
Similar articles
- Mutations at alternative 5' splice sites of M1 mRNA negatively affect influenza A virus viability and growth rate.
Chiang C, Chen GW, Shih SR. Chiang C, et al. J Virol. 2008 Nov;82(21):10873-86. doi: 10.1128/JVI.00506-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768984 Free PMC article. - An A14U Substitution in the 3' Noncoding Region of the M Segment of Viral RNA Supports Replication of Influenza Virus with an NS1 Deletion by Modulating Alternative Splicing of M Segment mRNAs.
Zheng M, Wang P, Song W, Lau SY, Liu S, Huang X, Mok BW, Liu YC, Chen Y, Yuen KY, Chen H. Zheng M, et al. J Virol. 2015 Oct;89(20):10273-85. doi: 10.1128/JVI.00919-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223635 Free PMC article. - Ecology and epidemiology of avian influenza in North and South America.
Senne DA, Suarez DL, Stallnecht DE, Pedersen JC, Panigrahy B. Senne DA, et al. Dev Biol (Basel). 2006;124:37-44. Dev Biol (Basel). 2006. PMID: 16447492 Review. - Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4.
Lee DH, Bertran K, Kwon JH, Swayne DE. Lee DH, et al. J Vet Sci. 2017 Aug 31;18(S1):269-280. doi: 10.4142/jvs.2017.18.S1.269. J Vet Sci. 2017. PMID: 28859267 Free PMC article. Review.
Cited by
- HLA-A*11:01-restricted CD8+ T cell immunity against influenza A and influenza B viruses in Indigenous and non-Indigenous people.
Habel JR, Nguyen AT, Rowntree LC, Szeto C, Mifsud NA, Clemens EB, Loh L, Chen W, Rockman S, Nelson J, Davies J, Miller A, Tong SYC, Rossjohn J, Gras S, Purcell AW, Hensen L, Kedzierska K, Illing PT. Habel JR, et al. PLoS Pathog. 2022 Mar 7;18(3):e1010337. doi: 10.1371/journal.ppat.1010337. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35255101 Free PMC article. - A LC3-interacting motif in the influenza A virus M2 protein is required to subvert autophagy and maintain virion stability.
Beale R, Wise H, Stuart A, Ravenhill BJ, Digard P, Randow F. Beale R, et al. Cell Host Microbe. 2014 Feb 12;15(2):239-47. doi: 10.1016/j.chom.2014.01.006. Cell Host Microbe. 2014. PMID: 24528869 Free PMC article. - Sequence characteristics and phylogenetic analysis of H9N2 subtype avian influenza A viruses detected from poultry and the environment in China, 2018.
Gao X, Wang N, Chen Y, Gu X, Huang Y, Liu Y, Jiang F, Bai J, Qi L, Xin S, Shi Y, Wang C, Liu Y. Gao X, et al. PeerJ. 2021 Dec 20;9:e12512. doi: 10.7717/peerj.12512. eCollection 2021. PeerJ. 2021. PMID: 35036116 Free PMC article. - Strand-Specific Dual RNA Sequencing of Bronchial Epithelial Cells Infected with Influenza A/H3N2 Viruses Reveals Splicing of Gene Segment 6 and Novel Host-Virus Interactions.
Fabozzi G, Oler AJ, Liu P, Chen Y, Mindaye S, Dolan MA, Kenney H, Gucek M, Zhu J, Rabin RL, Subbarao K. Fabozzi G, et al. J Virol. 2018 Aug 16;92(17):e00518-18. doi: 10.1128/JVI.00518-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29976658 Free PMC article. - Viral proteins that bind double-stranded RNA: countermeasures against host antiviral responses.
Krug RM. Krug RM. J Interferon Cytokine Res. 2014 Jun;34(6):464-8. doi: 10.1089/jir.2014.0005. J Interferon Cytokine Res. 2014. PMID: 24905203 Free PMC article. Review.
References
- Du L, Zhou Y, Jiang S (2010) Research and development of universal influenza vaccines. Microbes Infect 12: 280–286. - PubMed
- Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, et al. (2009) M2e-based universal influenza A vaccine. Vaccine 27: 6280–6283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 088789/Wellcome Trust/United Kingdom
- G0700815/MRC_/Medical Research Council/United Kingdom
- G0801931/MRC_/Medical Research Council/United Kingdom
- 075450/WT_/Wellcome Trust/United Kingdom
- ImNIH/Intramural NIH HHS/United States
- Wellcome Trust/United Kingdom
- G0700848/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources