GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice - PubMed (original) (raw)
doi: 10.1371/journal.pone.0049152. Epub 2012 Nov 2.
Yanan Wang, Janine J Geerling, Janny P Schröder-Van der Elst, Kristen Picha, Karyn O'Neil, Vedrana Stojanovic-Susulic, Tatiana Ort, Louis M Havekes, Johannes A Romijn, Hanno Pijl, Patrick C N Rensen
Affiliations
- PMID: 23133675
- PMCID: PMC3487842
- DOI: 10.1371/journal.pone.0049152
GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice
Edwin T Parlevliet et al. PLoS One. 2012.
Abstract
Objective: In addition to improve glucose intolerance, recent studies suggest that glucagon-like peptide-1 (GLP-1) receptor agonism also decreases triglyceride (TG) levels. The aim of this study was to evaluate the effect of GLP-1 receptor agonism on very-low-density lipoprotein (VLDL)-TG production and liver TG metabolism.
Experimental approach: The GLP-1 peptide analogues CNTO3649 and exendin-4 were continuously administered subcutaneously to high fat diet-fed APOE*3-Leiden transgenic mice. After 4 weeks, hepatic VLDL production, lipid content, and expression profiles of selected genes involved in lipid metabolism were determined.
Results: CNTO3649 and exendin-4 reduced fasting plasma glucose (up to -30% and -28% respectively) and insulin (-43% and -65% respectively). In addition, these agents reduced VLDL-TG production (-36% and -54% respectively) and VLDL-apoB production (-36% and -43% respectively), indicating reduced production of VLDL particles rather than reduced lipidation of apoB. Moreover, they markedly decreased hepatic content of TG (-39% and -55% respectively), cholesterol (-30% and -55% respectively), and phospholipids (-23% and -36% respectively), accompanied by down-regulation of expression of genes involved in hepatic lipogenesis (Srebp-1c, Fasn, Dgat1) and apoB synthesis (Apob).
Conclusion: GLP-1 receptor agonism reduces VLDL production and hepatic steatosis in addition to an improvement of glycemic control. These data suggest that GLP-receptor agonists could reduce hepatic steatosis and ameliorate dyslipidemia in patients with type 2 diabetes mellitus.
Conflict of interest statement
Competing Interests: This research was supported by a grant from Janssen Research & Development. The CNTO3649 used in this study is a Janssen product. There are no further patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
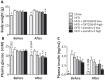
Figure 1. GLP-1 receptor agonism reduces body weight and fasting plasma glucose and insulin levels.
APOE*3-Leiden (E3L) mice were fed a high fat diet (HFD) for 22 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (1.0 or 3.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Blood was collected by tail bleeding after 7 h of fasting. Just before drug treatment (week 18) and after treatment (week 22), body weight (A), plasma glucose (B) and plasma insulin (C) levels were determined. Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01 compared to HFD controls.
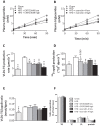
Figure 2. GLP-1 receptor agonism reduces hepatic VLDL-TG and VLDL-apoB production without affecting VLDL particle composition.
E3L mice were fed a HFD for 22 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (1.0 or 3.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice fed a chow diet was included that received vehicle (chow control). After 7 h fasting, mice were injected with Tran35S label (t = −30 min) and Triton WR-1339 (t = 0 min). Blood was drawn at the indicated time points and plasma TG concentrations were determined (A, B). VLDL-TG production rate was calculated as µmol/h from the slopes of the TG-time curves of the individual mice (C). At t = 120 min, mice were exsanguinated, and VLDL was isolated by density gradient ultracentrifugation. 35S-activity was determined, and VLDL-apoB production rate was calculated as dpm.h−1 (D). The VLDL-TG production rate to VLDL-apoB production rate ratio was calculated as nmol/dpm (E). The content of triglycerides, cholesterol, phospholipids and protein in VLDL was determined and calculated as % of total mass (F). Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls. TG: triglycerides; TC: total cholesterol; PL: phospholipids; Pro: protein.
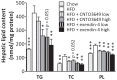
Figure 3. GLP-1 receptor agonism reverses high fat diet-induced hepatic steatosis.
E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Livers were isolated from 7 h fasted mice, liver pieces were homogenized, and triglycerides, cholesterol and phospholipids were determined as nmol per mg protein. Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls.
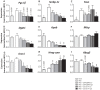
Figure 4. GLP-1 receptor agonism affects hepatic expression of genes involved in VLDL production, lipogenesis, and lipid homeostasis.
E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Livers were isolated from 7 h fasted mice, and mRNA was extracted from liver pieces. mRNA values of indicated genes were normalized to Cyclo and Hprt mRNA levels. Data were calculated as fold difference as compared with the HFD control group. Values are means ± SEM for at least 6 mice per group. *P<0.05, **P<0.01, ***P<0.001 compared to HFD controls.
Similar articles
- Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration.
Wang Y, Parlevliet ET, Geerling JJ, van der Tuin SJ, Zhang H, Bieghs V, Jawad AH, Shiri-Sverdlov R, Bot I, de Jager SC, Havekes LM, Romijn JA, Willems van Dijk K, Rensen PC. Wang Y, et al. Br J Pharmacol. 2014 Feb;171(3):723-34. doi: 10.1111/bph.12490. Br J Pharmacol. 2014. PMID: 24490861 Free PMC article. - GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance.
Khound R, Taher J, Baker C, Adeli K, Su Q. Khound R, et al. Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):2252-2259. doi: 10.1161/ATVBAHA.117.310251. Epub 2017 Oct 26. Arterioscler Thromb Vasc Biol. 2017. PMID: 29074588 - GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance.
Taher J, Baker CL, Cuizon C, Masoudpour H, Zhang R, Farr S, Naples M, Bourdon C, Pausova Z, Adeli K. Taher J, et al. Mol Metab. 2014 Sep 28;3(9):823-33. doi: 10.1016/j.molmet.2014.09.005. eCollection 2014 Dec. Mol Metab. 2014. PMID: 25506548 Free PMC article. - Effect of GLP-1 based therapies on diabetic dyslipidemia.
Patel VJ, Joharapurkar AA, Shah GB, Jain MR. Patel VJ, et al. Curr Diabetes Rev. 2014;10(4):238-50. doi: 10.2174/1573399810666140707092506. Curr Diabetes Rev. 2014. PMID: 24998439 Review. - New and emerging regulators of intestinal lipoprotein secretion.
Xiao C, Dash S, Morgantini C, Lewis GF. Xiao C, et al. Atherosclerosis. 2014 Apr;233(2):608-615. doi: 10.1016/j.atherosclerosis.2013.12.047. Epub 2014 Jan 21. Atherosclerosis. 2014. PMID: 24534456 Review.
Cited by
- Therapeutic Mechanisms and Clinical Effects of Glucagon-like Peptide 1 Receptor Agonists in Nonalcoholic Fatty Liver Disease.
Lee HA, Kim HY. Lee HA, et al. Int J Mol Sci. 2023 May 26;24(11):9324. doi: 10.3390/ijms24119324. Int J Mol Sci. 2023. PMID: 37298276 Free PMC article. Review. - Lipoprotein effects of incretin analogs and dipeptidyl peptidase 4 inhibitors.
Zhong J, Maiseyeu A, Rajagopalan S. Zhong J, et al. Clin Lipidol. 2015;10(1):103-112. doi: 10.2217/clp.14.59. Clin Lipidol. 2015. PMID: 26005496 Free PMC article. - Glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: An update.
Sofogianni A, Filippidis A, Chrysavgis L, Tziomalos K, Cholongitas E. Sofogianni A, et al. World J Hepatol. 2020 Aug 27;12(8):493-505. doi: 10.4254/wjh.v12.i8.493. World J Hepatol. 2020. PMID: 32952876 Free PMC article. Review. - The effects of exendin-4 treatment on graft failure: an animal study using a novel re-vascularized minimal human islet transplant model.
Sahraoui A, Winzell MS, Gorman T, Smith DM, Skrtic S, Hoeyem M, Abadpour S, Johansson L, Korsgren O, Foss A, Scholz H. Sahraoui A, et al. PLoS One. 2015 Mar 20;10(3):e0121204. doi: 10.1371/journal.pone.0121204. eCollection 2015. PLoS One. 2015. PMID: 25793295 Free PMC article. - Exendin-4 as a Versatile Therapeutic Agent for the Amelioration of Diabetic Changes.
Rajabi H, Ahmadi M, Aslani S, Saberianpour S, Rahbarghazi R. Rajabi H, et al. Adv Pharm Bull. 2022 Mar;12(2):237-247. doi: 10.34172/apb.2022.025. Epub 2021 Jan 31. Adv Pharm Bull. 2022. PMID: 35620334 Free PMC article. Review.
References
- Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365: 1333–1346. - PubMed
- Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846. - PubMed
- Lewis JR, Mohanty SR (2010) Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 55: 560–578. - PubMed
- Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2: 1300–1304. - PubMed
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C (1997) Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous