Divergence of the systemic immune response following oral infection with distinct strains of Porphyromonas gingivalis - PubMed (original) (raw)
Divergence of the systemic immune response following oral infection with distinct strains of Porphyromonas gingivalis
J T Marchesan et al. Mol Oral Microbiol. 2012 Dec.
Abstract
Periodontitis is a polymicrobial oral infection characterized by the destruction of tooth-supporting structures that can be linked to systemic diseases such as cardiovascular disease, diabetes or rheumatoid arthritis. Porphyromonas gingivalis, a bacterium implicated in the etiology of periodontitis, has shown variation in inducing T-cell responses among different strains. Therefore, in this study we investigated the strain-specific immune response using a murine experimental model of periodontitis. Periodontitis was induced by P. gingivalis strains A7A1-28, W83 and W50, and later confirmed by the presence of P. gingivalis in the oral microflora and by alveolar bone resorption. Splenocytes were evaluated for gene expression, cellular proteins and cytokine expression. Dendritic cells were stimulated in vitro for T helper cell-cytokine profiling. Results showed that P. gingivalis had the ability to alter the systemic immune response after bacterial exposure. Strains W50 and W83 were shown to induce alveolar bone loss, whereas the A7A1-28 strain did not significantly promote bone resorption in mice. Splenocytes derived from mice infected with strains W50 and W83 induced expression of high levels of interleukin-4 (IL-4) but A7A1-28 stimulated increased IL-10. Stimulation of dendritic cells in vitro showed a similar pattern of cytokine expression of IL-12p40, IL-6 and transforming growth factor-β among strains. A distinct systemic response in vivo was observed among different strains of P. gingivalis, with IL-10 associated with the least amount of alveolar bone loss. Evaluation of pathogen-driven systemic immune responses associated with periodontal disease pathogenesis may assist in defining how periodontitis may impact other diseases.
© 2012 John Wiley & Sons A/S.
Figures
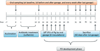
Figure 1
Schematic diagram illustrating the study experimental design. This timeline displays the in vivo experiment, including mice acclimation, antibiotic treatment, Porphyromonas gingivalis (Pg) infection period and periodontitis developmental (PD) phase, oral microflora sampling, spleen harvest and sacrifice.
Figure 2
Polymerase chain reaction (PCR) analysis of Porphyromonas gingivalis oral infection determined by Arg-gingipain (201 bp). (A) Confirmation of P. gingivalis colonies of strains W83, W50 and A7A1-28 before gavage. (B) Porphyromonas gingivalis colony-forming units (CFU) at 0, 102, 103 and 104 were added to a sample collected from the oral microflora of mice to determine the detection limit via PCR. Oral microflora analysis: samples of mice infected with P. gingivalis at baseline (upper panel) and at 42 days after the first gavage (lower panel). Numbers 1 to 8 represent individual mice gavaged with (C) vehicle alone, (D) A7A1-28, (E) W83 and (F) W50.
Figure 3
Alveolar bone loss measured by micro-computed tomography. (A) Representative images of the alveolar bone loss found in mice at 42 days after gavage was performed with the three different strains A7A1-28, W83 and W50. (B) Analysis performed for tissue mineral content, bone mineral content, bone volume and bone volume fraction. Error bars indicate standard error. Asterisks denote differences found among groups by Tukey test (P < 0.05).
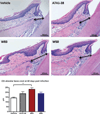
Figure 4
Histomorphometric image analysis performed in maxillae sections of mice at 42 days after gavage; the distance between the alveolar bone crest to the cementum–enamel junction in M1 was measured in micrometers (depicted with black arrows) (hematoxylin & eosin, 20×)
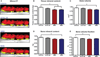
Figure 5
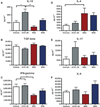
Flow cytometric analysis of splenocytes derived from mice at 42 days after gavage with Porphyromonas gingivalis for regulatory markers. (A) CD3+ CD4+ Foxp3+ cell percentage, (B) CD3+ CD4+ IL-10+ cell percentage, (C) mRNA FasL relative expression of non-activated splenocytes (towards GAPDH), and (D) CD5+ B220+ cell percentage. Error bars indicate standard error. Asterisks denote differences found among groups by Tukey test (*P < 0.05, **P < 0.001).
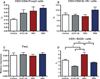
Figure 6
Cytokine expression of 42 days post-gavage murine splenocytes treated with phorbol 12-myristate 13-acetate (PMA)/ionomycin for 48 h in vitro. (A) Differences were found among groups for the expression of interleukin-10 (IL-10; P = 0.0006) and no differences for (B) transforming growth factor- β (TGF-β); (C) interferon-γ (IFN-γ) expression was higher in A7A1-28 strain, and (D) IL-4 expression was higher in W83 strain. No differences were found in expression of (E) IL-17 or (F) IL-6. Experiments were performed in duplicates. Error bars indicate standard error. Asterisks denote differences found among groups by Tukey test (*P < 0.05, **P < 0.001).
Figure 7
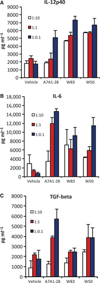
Dendritic cells stimulated with Porphyromonas gingivalis strains A7A1-28, W83 and W50 at multiplicities of infection 1:10, 1:1, 1 : 0.1 showed an expression of (A) interleukin-12 (IL-12) p40, (B) IL-6 and (C) transforming growth factor-β (TGF-β) in a dose-dependent manner. Experiments were performed in duplicates. Error bars indicate standard error.
Similar articles
- Natural killer T cells mediate alveolar bone resorption and a systemic inflammatory response in response to oral infection of mice with Porphyromonas gingivalis.
Aoki-Nonaka Y, Nakajima T, Miyauchi S, Miyazawa H, Yamada H, Domon H, Tabeta K, Yamazaki K. Aoki-Nonaka Y, et al. J Periodontal Res. 2014 Feb;49(1):69-76. doi: 10.1111/jre.12080. Epub 2013 Apr 16. J Periodontal Res. 2014. PMID: 23586756 - T cell response mediated by myeloid cell-derived IL-12 is responsible for Porphyromonas gingivalis-induced periodontitis in IL-10-deficient mice.
Sasaki H, Suzuki N, Kent R Jr, Kawashima N, Takeda J, Stashenko P. Sasaki H, et al. J Immunol. 2008 May 1;180(9):6193-8. doi: 10.4049/jimmunol.180.9.6193. J Immunol. 2008. PMID: 18424741 - Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo.
Hajishengallis G, Shakhatreh MA, Wang M, Liang S. Hajishengallis G, et al. J Immunol. 2007 Aug 15;179(4):2359-67. doi: 10.4049/jimmunol.179.4.2359. J Immunol. 2007. PMID: 17675497 - Dendritic cells a critical link to alveolar bone loss and systemic disease risk in periodontitis: Immunotherapeutic implications.
El-Awady AR, Elashiry M, Morandini AC, Meghil MM, Cutler CW. El-Awady AR, et al. Periodontol 2000. 2022 Jun;89(1):41-50. doi: 10.1111/prd.12428. Epub 2022 Mar 4. Periodontol 2000. 2022. PMID: 35244951 Free PMC article. Review. - Porphyromonas gingivalis: major periodontopathic pathogen overview.
Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, Prochazkova J, Duskova J. Mysak J, et al. J Immunol Res. 2014;2014:476068. doi: 10.1155/2014/476068. Epub 2014 Mar 25. J Immunol Res. 2014. PMID: 24741603 Free PMC article. Review.
Cited by
- Distinctive pathways characterize A. actinomycetemcomitans and P. gingivalis.
Lv J, Zhu YX, Liu YQ, Xue X. Lv J, et al. Mol Biol Rep. 2015 Feb;42(2):441-9. doi: 10.1007/s11033-014-3785-2. Epub 2014 Oct 29. Mol Biol Rep. 2015. PMID: 25351486 Retracted. - Interplay between P. gingivalis, F. nucleatum and A. actinomycetemcomitans in murine alveolar bone loss, arthritis onset and progression.
Ebbers M, Lübcke PM, Volzke J, Kriebel K, Hieke C, Engelmann R, Lang H, Kreikemeyer B, Müller-Hilke B. Ebbers M, et al. Sci Rep. 2018 Oct 11;8(1):15129. doi: 10.1038/s41598-018-33129-z. Sci Rep. 2018. PMID: 30310087 Free PMC article. - Oral biofilm dysbiosis during experimental periodontitis.
Ribeiro AA, Jiao Y, Girnary M, Alves T, Chen L, Farrell A, Wu D, Teles F, Inohara N, Swanson KV, Marchesan JT. Ribeiro AA, et al. Mol Oral Microbiol. 2022 Dec;37(6):256-265. doi: 10.1111/omi.12389. Epub 2022 Oct 19. Mol Oral Microbiol. 2022. PMID: 36189827 Free PMC article. - Porphyromonas gingivalis within Placental Villous Mesenchyme and Umbilical Cord Stroma Is Associated with Adverse Pregnancy Outcome.
Vanterpool SF, Been JV, Houben ML, Nikkels PG, De Krijger RR, Zimmermann LJ, Kramer BW, Progulske-Fox A, Reyes L. Vanterpool SF, et al. PLoS One. 2016 Jan 5;11(1):e0146157. doi: 10.1371/journal.pone.0146157. eCollection 2016. PLoS One. 2016. PMID: 26731111 Free PMC article. - Bone loss and aggravated autoimmune arthritis in HLA-DRβ1-bearing humanized mice following oral challenge with Porphyromonas gingivalis.
Sandal I, Karydis A, Luo J, Prislovsky A, Whittington KB, Rosloniec EF, Dong C, Novack DV, Mydel P, Zheng SG, Radic MZ, Brand DD. Sandal I, et al. Arthritis Res Ther. 2016 Oct 26;18(1):249. doi: 10.1186/s13075-016-1143-6. Arthritis Res Ther. 2016. PMID: 27784339 Free PMC article.
References
- Albandar JM. Underestimation of periodontitis in NHANES surveys. J Periodontal. 2011;82:337–341. - PubMed
- Baker PJ, Dixon M, Evans RT, Roopenian DC. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol Immunol. 2000;15:27–32. - PubMed
- Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 2009;24:469–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources