Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity - PubMed (original) (raw)
Review
Structure and function of the SPRY/B30.2 domain proteins involved in innate immunity
Akshay A D'Cruz et al. Protein Sci. 2013 Jan.
Abstract
The SPRY domain is a protein interaction module found in 77 murine and ~100 human proteins, and is implicated in important biological pathways, including those that regulate innate and adaptive immunity. The current definition of the SPRY domain is based on a sequence repeat discovered in the splA kinase and ryanodine receptors. The greater SPRY family is divided into the B30.2 (which contains a PRY extension at the N-terminus) and "SPRY-only" sub-families. In this brief review, we examine the current structural and biochemical literature on SPRY/B30.2 domain involvement in key immune processes and highlight a PRY-like 60 amino acid region in the N-terminus of "SPRY-only" proteins. Phylogenetic, structural, and functional analyses suggest that this N-terminal region is related to the PRY region of B30.2 and should be characterized as part of an extended SPRY domain. Greater understanding of the functional importance of the N-terminal region in "SPRY only" proteins will enhance our ability to interrogate SPRY interactions with their respective binding partners.
Copyright © 2012 The Protein Society.
Figures
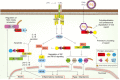
Figure 1
The role of SPRY/B30.2 domain-containing proteins in innate immune responses. Activation of Toll-like receptor (TLR) 4 signaling by pathogen-associated molecular patterns (PAMP) such as lipopolysaccharide (LPS) occurs via MyD88 (myeloid differentiation primary response protein)–dependent and independent pathways, the latter via the TRIF (TIR domain-containing adapter molecule 1) complex. Both pathways activate NEMO (NFκB essential modulator, also known as IKKγ), which in turn activates the IKK complex (IκB kinases, α and β), which inhibits IκB (inhibitor of NF-κB), releasing NFκB (nuclear factor-κB) for translocation into the nucleus. The NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome activates caspase-1 in an ASC (apoptosis-associated speck-like protein containing a CARD)-dependent manner, which then processes pro-IL-1β and pro-IL-18 into their active forms. The B30.2 domain of pyrin interacts with caspase-1 to regulate the cleavage of pro-IL-1β and pro-IL-18. NEMO also activates the IKKε, TANK1 (TRAF family member-associated NF-kappa-B activator 1), TBK1 (TANK-binding kinase 1) complex, leading to phosphorylation- and dimerization-mediated activation of the interferon regulatory factors (IRFs) and transcription of target genes. RING, Really Interesting New Gene; PYD, pyrin domain; CCD, coiled-coil domain; SOCS box, suppressor of cytokine signaling.
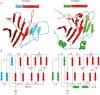
Figure 2
Structural conservation of the PRY region of TRIM21 and the N-terminal region of SPSB2 suggests these regions are evolutionarily and functionally related. A: Cartoon representation of the TRIM21 B30.2 domain283–465 (PDB: 2VOK) and SPSB2 SPRY domain12–219 (PDB: 3EK9) crystal structures. The SPRY regions of both proteins are in red, while the PRY and N-terminal regions are in cyan and green, respectively. The amino acid numbers and domain architectures are shown. B: Topology diagrams of the TRIM21 B30.2 (left) and SPSB2 SPRY (right) domains with the structural elements colored as in (A). α-helices and β-strands are numbered, and shown as rounded rectangles and arrows, respectively. Loop regions are numbered L1–L13. Based on comparisons with all SPRY structures to date, we consider the first beta strand of SPSB2 as too distorted to be a true secondary element, therefore the N-terminal region is shown here as containing 3 β-strands.
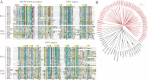
Figure 3
A: Sequence alignment of select members of the murine B30.2 and “SPRY-only” domain family. Sequences were selected from across the SPRY/B30.2 family, sourced from Genbank and aligned using ClustalX 2.0.12. Secondary structural elements positioned above the alignment correspond to residues from the TRIM21 crystal structure.75 The BLACK line denotes the beginning of the SPRY region. An extra α-helix (denoted by *) was resolved at the N-terminus of human TRIM72 and murine SPSB2.9,86 B: The B30.2 sub-family clusters together and shares a common ancestral gene. Phylogenetic tree constructed from B30.2 (PRY and SPRY) and “SPRY-only” (N-terminal and SPRY) sequences sourced from Genbank and aligned using ClustalX 2.0.12. An unrooted phylogenetic tree was then generated using Quicktree v1.1. The scale refers to the number of millions of years since the sequences last shared a common ancestor. The RYR family members each contain three SPRY domains, which are distinguished by their relative location within the protein domain architecture, that is, “SPRY1” denotes the most N-terminal of the SPRY domains, with “SPRY2” in the middle and “SPRY3” nearest to the C-terminus. SPRYD3 proteins contain two SPRY domains and are labeled as SPRY1 and SPRY2, respectively. All sequences are from mouse, with the exception of pyrin and TRIM72, for which the human sequence was used.
Similar articles
- Dynamics of the SPRY domain-containing SOCS box protein 2: flexibility of key functional loops.
Yao S, Liu MS, Masters SL, Zhang JG, Babon JJ, Nicola NA, Nicholson SE, Norton RS. Yao S, et al. Protein Sci. 2006 Dec;15(12):2761-72. doi: 10.1110/ps.062477806. Epub 2006 Nov 6. Protein Sci. 2006. PMID: 17088318 Free PMC article. - Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence?
Rhodes DA, de Bono B, Trowsdale J. Rhodes DA, et al. Immunology. 2005 Dec;116(4):411-7. doi: 10.1111/j.1365-2567.2005.02248.x. Immunology. 2005. PMID: 16313355 Free PMC article. Review. - Exploring the diversity of SPRY/B30.2-mediated interactions.
Perfetto L, Gherardini PF, Davey NE, Diella F, Helmer-Citterich M, Cesareni G. Perfetto L, et al. Trends Biochem Sci. 2013 Jan;38(1):38-46. doi: 10.1016/j.tibs.2012.10.001. Epub 2012 Nov 17. Trends Biochem Sci. 2013. PMID: 23164942 Review. - Backbone 1H, 13C, and 15N resonance assignments of the PRY-SPRY domain of RNF135.
Zhang D, Wei H, Xue H, Guo S, Wu B, Kuang Z. Zhang D, et al. Biomol NMR Assign. 2019 Oct;13(2):299-304. doi: 10.1007/s12104-019-09895-w. Epub 2019 May 7. Biomol NMR Assign. 2019. PMID: 31065957 - Crystal structure of the TRIM25 B30.2 (PRYSPRY) domain: a key component of antiviral signalling.
D'Cruz AA, Kershaw NJ, Chiang JJ, Wang MK, Nicola NA, Babon JJ, Gack MU, Nicholson SE. D'Cruz AA, et al. Biochem J. 2013 Dec 1;456(2):231-40. doi: 10.1042/BJ20121425. Biochem J. 2013. PMID: 24015671 Free PMC article.
Cited by
- Bacterial death and TRADD-N domains help define novel apoptosis and immunity mechanisms shared by prokaryotes and metazoans.
Kaur G, Iyer LM, Burroughs AM, Aravind L. Kaur G, et al. Elife. 2021 Jun 1;10:e70394. doi: 10.7554/eLife.70394. Elife. 2021. PMID: 34061031 Free PMC article. - TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy.
Jena KK, Kolapalli SP, Mehto S, Nath P, Das B, Sahoo PK, Ahad A, Syed GH, Raghav SK, Senapati S, Chauhan S, Chauhan S. Jena KK, et al. EMBO J. 2018 Sep 14;37(18):e98358. doi: 10.15252/embj.201798358. Epub 2018 Aug 24. EMBO J. 2018. PMID: 30143514 Free PMC article. - Crystal structure of the SPRY domain of human SPSB2 in the apo state.
Luo Y, Li K, Yang J, Zhang D, Zhou Y, Kuang Z. Luo Y, et al. Acta Crystallogr F Struct Biol Commun. 2019 Jun 1;75(Pt 6):412-418. doi: 10.1107/S2053230X1900623X. Epub 2019 May 10. Acta Crystallogr F Struct Biol Commun. 2019. PMID: 31204687 Free PMC article. - Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells.
Yuan L, Ma X, Yang Y, Qu Y, Li X, Zhu X, Ma W, Duan J, Xue J, Yang H, Huang JW, Yi S, Zhang M, Cai N, Zhang L, Ding Q, Lai K, Liu C, Zhang L, Liu X, Yao Y, Zhou S, Li X, Shen P, Chang Q, Malwal SR, He Y, Li W, Chen C, Chen CC, Oldfield E, Guo RT, Zhang Y. Yuan L, et al. Nature. 2023 Sep;621(7980):840-848. doi: 10.1038/s41586-023-06525-3. Epub 2023 Sep 6. Nature. 2023. PMID: 37674084 Free PMC article. - Molecular cloning, characterization and expression profiling of a ryanodine receptor gene in Asian corn borer, Ostrinia furnacalis (Guenée).
Cui L, Yang D, Yan X, Rui C, Wang Z, Yuan H. Cui L, et al. PLoS One. 2013 Oct 1;8(10):e75825. doi: 10.1371/journal.pone.0075825. eCollection 2013. PLoS One. 2013. PMID: 24098400 Free PMC article.
References
- Vernet C, Boretto J, Mattei MG, Takahashi M, Jack LJ, Mather IH, Rouquier S, Pantarotti P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J Mol Evol. 1993;37:600–612. - PubMed
- Henry J, Ribouchon MT, Offer C, Pontarotti P. B30.2-like domain proteins: a growing family. Biochem Biophys Res Commun. 1997;235:162–165. - PubMed
- Ponting C, Schultz J, Bork P. SPRY domains in ryanodine receptors (Ca(2+)-release channels. Trends Biochem Sci. 1997;22:193–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources