Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes - PubMed (original) (raw)
Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-II-transcribed genes
Amaya Alzu et al. Cell. 2012.
Abstract
Transcription hinders replication fork progression and stability. The ATR checkpoint and specialized DNA helicases assist DNA synthesis across transcription units to protect genome integrity. Combining genomic and genetic approaches together with the analysis of replication intermediates, we searched for factors coordinating replication with transcription. We show that the Sen1/Senataxin DNA/RNA helicase associates with forks, promoting their progression across RNA polymerase II (RNAPII)-transcribed genes. sen1 mutants accumulate aberrant DNA structures and DNA-RNA hybrids while forks clash head-on with RNAPII transcription units. These replication defects correlate with hyperrecombination and checkpoint activation in sen1 mutants. The Sen1 function at the forks is separable from its role in RNA processing. Our data, besides unmasking a key role for Senataxin in coordinating replication with transcription, provide a framework for understanding the pathological mechanisms caused by Senataxin deficiencies and leading to the severe neurodegenerative diseases ataxia with oculomotor apraxia type 2 and amyotrophic lateral sclerosis 4.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
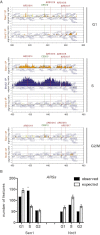
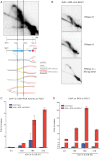
Genome-wide Analysis of Sen1 and Nrd1 Localization during the Cell Cycle (A) _SEN1_- (Sen1) and NRD1-FLAG (Nrd1) strains were blocked in G1 phase by α-factor treatment (G1) and were released at 23°C in either 0.2 M HU for 60 min (S phase; S) or nocodazole for 180 min (G2/M). Samples were collected in each cell-cycle phase to analyze the genomic distribution of factors by ChIP-chip. The number of features bound by each factor in the ChIP experiments (observed) as well as in bioinformatic simulations is shown for highly transcribed RNAPII genes, snoRNAs, RNAPIII genes, and centromeres (see Experimental Procedures). Data represent the mean ±SD of three independent simulations (expected). Asterisks indicate features showing a statistically significant difference between observed and simulated binding. The statistical significance of Sen1 and Nrd1 binding to centromeres could not be calculated by this method due to the reduced total number—sixteen—of these elements. (B) WT and rpb1-1 cells were arrested in G2/M phase as in Figure 1A, transferred to 37°C for 1 hr to inactivate the RNAPII, and then processed for ChIP-chip to analyze Sen1 binding. (C) Sen1 binding to a WT or a B-block promoter mutant tE(CUC)D gene was evaluated by ChIP, followed by qPCR in G2/M-arrested cells. Data represent the mean ±SD of three independent experiments. (D) Serial dilutions of WT, sen1-1, and nrd1-102 cells plated in the presence or absence (UNT) of benomyl. See also Figure S1.
Figure 2
Sen1 Localizes at Replication Forks (A) Sen1 and Nrd1 distributions at replication origins by ChIP-chip throughout the cell cycle. Cells were synchronized in G1, S, and G2/M phases, and Sen1 and Nrd1 binding profiles were obtained as in Figure 1A. Blue (BrdU-IP) histogram bars in the y axis show the average signal ratio in log2 scale on loci along the reported region on chromosome X in S-phase-arrested cells. Replication origin, ORF, and CEN10 sequences are indicated. (B) The number of features bound by each factor in the ChIP experiments (observed), as well as in bioinformatic simulations, is shown for ARS elements (see Experimental Procedures). Data represent the mean ±SD of three independent simulations (expected). Asterisks indicate features showing a statistically significant difference between observed and simulated binding. See also Figure S2.
Figure 3
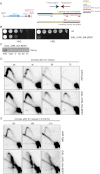
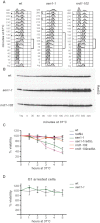
Sen1-Depleted Cells Exhibit Fork Defects at Highly Expressed RNAPII Genes PDC1-TRX1 (A) Schematic representation of 2D gel restriction strategy (left) and replication-transcription directionality at the PDC1-TRX1 locus (right). (B) Serial dilutions of WT and conditional lethal GAL::URL-HA-SEN1 strains were spotted on glucose (YPD)- or galactose (YPG)-containing media. (C–E) WT and GAL::URL-HA-SEN1 cells were grown in YPG, transferred to YPD for 1 hr to switch off SEN1, presynchronized in G1, and released into cell cycle in YPD with or without 0.2 M HU. Cells were collected to assess Sen1 protein levels (C) and replication intermediates by 2D gel analysis in the absence (D) or presence of HU (E). See also Figure S3.
Figure 4
Gapped Forks and DNA-RNA Hybrid Accumulation at the PDC1-TRX1 Locus in Sen1-Depleted Cells (A) Scheme, scaled on a representative 2D gel sen1 cells profile (Figure 3E), showing replication fork progression across the SphI restriction fragment containing the PDC1-TRX1 genes. The putative intermediates are shown. RNA (dashed blue line) in DNA-RNA hybrids was removed by RNase treatment prior the 2D gel analysis. Yellow lines mark the gapped regions on Ys intermediates predicted on the base of the sen1 2D gel profiles (see text for details). (B) RNases and Mung Bean nuclease sensitivity analysis on sen1 aberrant replication intermediates at 150 min in HU. (C and D) (C) DNA-RNA hybrids and RFA1-PK (Rfa1) (D) accumulation at PDC1 locus by ChIP followed by qPCR analysis in WT and GAL::URL-HA-SEN1 cells treated as in Figure 3E. Data represent the mean ±SD of three independent experiments. See also Figure S4.
Figure 5
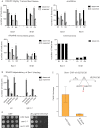
Replication Defects and DNA Damage Checkpoint Activation in sen1 Mutants (A–D) WT, sen1-1, and nrd1-102 cells were released from G1 phase arrest into the cell cycle at 37°C. At the indicated time points, cell samples were processed for FACS analysis to measure the DNA content (A) or TCA protein precipitation to detect Rad53 protein (B). Brackets indicate the extent of S phase. Rad53 phosphorylated isoforms are indicated by an asterisk. Survival curves of the indicated yeast strains after release from G1 phase into cell cycle at 37°C (C) or blocked in G1 at 37°C (D). Data represent the mean ±SD of three independent experiments. See also Figure S5.
Figure 6
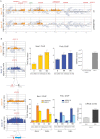
Replication/Recombination-Dependent Genotoxicity in sen1-1 Mutants (A–D) (A) SEN1 synthetic lethality map generated by Osprey (B) survival curves of the indicated yeast strains after release from G1 phase into cell cycle at 37°C. Tetrads obtained from sporulation of diploids heterozygous for the indicated mutations were grown at 25°C. Gene conversion and pop-out rates (C) and chromosome loss frequency (D) were determined for WT, sen1-1, and nrd1-102 strains at 30°C. Data in (B) and (C) represent the mean ±SD of three independent experiments. An example of red/white sector phenotype in sen1-1 mutants is shown in (D). See also Figure S6.
Figure 7
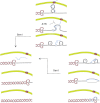
Model for DNA-RNA Hybrid Accumulation in sen1 Mutants at Sites of Collision between Replication and Transcription The ATR checkpoint inhibits gene gating to allow fork progression through topological barriers imposed by RNAPII-transcribed genes. Following gene loop dismantlement, twin-supercoiled domains transiently form, and nascent transcripts may anneal to the negatively supercoiled DNA behind the transcription bubble, leading to DNA-RNA hybrid formation. Sen1 moves with the fork, preventing DNA-RNA hybrid accumulation at those genomic regions at which transcription collides head-on with replication. In the absence of Sen1, DNA-RNA hybrids persist on the lagging strand template. Replication across the nontranscribed strand might be facilitated by the uncoupling of leading- and lagging-strand synthesis.
Figure S1
Genome-wide Distribution of Sen1 and Nrd1 and Sen1 Binding Dependency on RNAPII Activity, Related to Figure 1 Examples of Nrd1 and Sen1 bound loci in G2/M phase arrested cells and Sen1 binding to the same loci in rpb1-1 mutants. Experiments are described in Figure 1. Orange histogram bars in the y axis show the average signal ratio in log2 scale of loci significantly enriched in the IP fractions along the indicated regions. The x axis show chromosomal coordinates at representative DNA genomic regions containing the indicated genomic features.
Figure S2
Analysis of Sen1 Origin Binding Dependency on Replication and Transcription, Related to Figure 2 (A) Sen1 ChIP-chip analysis in WT and disARS607 origin mutant cells 60 min after release from G1 phase into 0.2 M HU. Orange (Sen1-IP) histogram bars in the y axis show the average signal log ratio of loci significantly enriched in the IP fractions along the indicated regions on chromosome VI. (B) Sen1 and BrdU chromatin distributions at ARS718 and ARS416 replication origins by ChIP-chip are shown on the left. SEN1-FLAG (Sen1) and POL1-MYC (Pol α) strains were arrested in G1 by α-factor treatment and released into cell cycle at 23°C in 0.2 M HU. The occupancy profiles of Sen1 and Pol α at a non-transcribed DNA region close to ARS718 and at ARS416-GAL3 locus were analyzed by ChIP-qPCR in G1- and S-phases. Data represent the mean ± SD of three independent experiments. The position of oligoes used in QPCR is indicated in the schemes under the ChIP-chip maps. At ARS416-GAL3 locus, Sen1 and Pol α chromatin binding was analyzed in media containing either glucose (GLU) or galactose (GAL) to modulate GAL3 transcription. Pol α was used as a marker of origin firing (Lucca et al., 2004). mRNA levels were measured by qPCR. Data represent the mean ± SD of three independent experiments.
Figure S3
BrdU Incorporation Analysis in WT and sen1-1 Mutants, Related to Figure 3 (A) BrdU-IP-Chip analysis of replicon dynamics in WT and sen1-1 cells 90 min after release from G1 into S-phase at 37°C in 0.2 M HU. Blue histogram peaks indicate the significant enrichment in the BrdU-IP fractions. Replication origin positions are indicated by red vertical bars. The Venn diagram shows the relative numbers of early origins fired in WT and sen1-1 cells. (B) 2D gel analysis of replication intermediates in WT and sen1-1 cells at the early ARS1 and middle/late ARS440 origins after release from G1 into S-phase at 37°C in 50 mM HU. 2D gels at ARS440 locus show a different exposure than those in Figure S5. Bubble arcs (black arrows) indicate that origins are fired. Spots on the Y arc in sen1-1 cells correspond to replication fork pause signals accumulating at SNR13-TRS31 genes (see text for details). (C) BrdU-IP-Chip analysis of replicon dynamics of WT and sen1-1 cells (orange and blue histogram bars, respectively) at PDC1-TRX1 locus in experimental conditions described in panel A. Vertical bars indicate the region showing fork slow-down in sen1-1 cells. In Figures 3 and 4, the ARS1211-PDC1-TRX1 locus is showed in a reversed orientation so that in the schemes and in the 2D gels fork movement is indicated in the same direction.
Figure S4
2D Gel Profiles at Sites of Collision between Replication Forks and RNAPII- or RNAPIII-Transcribed Genes, Related to Figure 4 WT and GAL::URL-HA-SEN1 cells were treated as described in Figure 3E and replication intermediates were analyzed by 2D gel analysis using probes recognizing the indicated restriction fragments. Brackets indicate fork pausing at regions experiencing head-on collisions between replication and RNAPII-dependent transcription in Sen1-depleted cells.
Figure S5
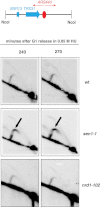
sen1-1, but Not nrd1-102, Mutants Exhibit Fork Pausing at the SNR13-TRS31 RNAPII-Transcribed Locus, Related to Figure 5 WT, sen1-1 and nrd1-102 cells were arrested in G1 phase and released into S-phase at 37°C in the presence of 50 mM HU. Replication intermediates were analyzed by 2D gel analysis using a probe recognizing the indicated restriction fragment. Arrows indicate fork pausing in sen1-1 mutants.
Figure S6
Synthetic Lethal Interactions between sen1-1 and Mutants Promoting Fork Instability, Related to Figure 6 Tetrads obtained from the sporulation of diploids heterozygous for the indicated double mutations were grown at 30°C. White circles indicate double mutant spores.
Similar articles
- Dormant origins and fork protection mechanisms rescue sister forks arrested by transcription.
Brambati A, Zardoni L, Achar YJ, Piccini D, Galanti L, Colosio A, Foiani M, Liberi G. Brambati A, et al. Nucleic Acids Res. 2018 Feb 16;46(3):1227-1239. doi: 10.1093/nar/gkx945. Nucleic Acids Res. 2018. PMID: 29059325 Free PMC article. - Sen1 Is Recruited to Replication Forks via Ctf4 and Mrc1 and Promotes Genome Stability.
Appanah R, Lones EC, Aiello U, Libri D, De Piccoli G. Appanah R, et al. Cell Rep. 2020 Feb 18;30(7):2094-2105.e9. doi: 10.1016/j.celrep.2020.01.087. Cell Rep. 2020. PMID: 32075754 Free PMC article. - Elongating RNA polymerase II and RNA:DNA hybrids hinder fork progression and gene expression at sites of head-on replication-transcription collisions.
Zardoni L, Nardini E, Brambati A, Lucca C, Choudhary R, Loperfido F, Sabbioneda S, Liberi G. Zardoni L, et al. Nucleic Acids Res. 2021 Dec 16;49(22):12769-12784. doi: 10.1093/nar/gkab1146. Nucleic Acids Res. 2021. PMID: 34878142 Free PMC article. - SUMOylated Senataxin functions in genome stability, RNA degradation, and stress granule disassembly, and is linked with inherited ataxia and motor neuron disease.
Bennett CL, La Spada AR. Bennett CL, et al. Mol Genet Genomic Med. 2021 Dec;9(12):e1745. doi: 10.1002/mgg3.1745. Epub 2021 Jul 14. Mol Genet Genomic Med. 2021. PMID: 34263556 Free PMC article. Review. - Senataxin: A key actor in RNA metabolism, genome integrity and neurodegeneration.
Giannini M, Porrua O. Giannini M, et al. Biochimie. 2024 Feb;217:10-19. doi: 10.1016/j.biochi.2023.08.001. Epub 2023 Aug 7. Biochimie. 2024. PMID: 37558082 Review.
Cited by
- Regulation of R-Loops in DNA Tumor Viruses.
Crowner A, Smith K, DeSmet M. Crowner A, et al. Pathogens. 2024 Oct 2;13(10):863. doi: 10.3390/pathogens13100863. Pathogens. 2024. PMID: 39452734 Free PMC article. - Transcription-replication conflicts: how they occur and how they are resolved.
García-Muse T, Aguilera A. García-Muse T, et al. Nat Rev Mol Cell Biol. 2016 Sep;17(9):553-63. doi: 10.1038/nrm.2016.88. Epub 2016 Jul 20. Nat Rev Mol Cell Biol. 2016. PMID: 27435505 Review. - Protective Mechanisms Against DNA Replication Stress in the Nervous System.
Charlier CF, Martins RAP. Charlier CF, et al. Genes (Basel). 2020 Jun 30;11(7):730. doi: 10.3390/genes11070730. Genes (Basel). 2020. PMID: 32630049 Free PMC article. Review. - Termination of Transcription of Short Noncoding RNAs by RNA Polymerase II.
Arndt KM, Reines D. Arndt KM, et al. Annu Rev Biochem. 2015;84:381-404. doi: 10.1146/annurev-biochem-060614-034457. Epub 2015 Mar 26. Annu Rev Biochem. 2015. PMID: 25747400 Free PMC article. Review. - Untangling the crosstalk between BRCA1 and R-loops during DNA repair.
San Martin Alonso M, Noordermeer SM. San Martin Alonso M, et al. Nucleic Acids Res. 2021 May 21;49(9):4848-4863. doi: 10.1093/nar/gkab178. Nucleic Acids Res. 2021. PMID: 33755171 Free PMC article. Review.
References
- Aguilera A. mRNA processing and genomic instability. Nat. Struct. Mol. Biol. 2005;12:737–738. - PubMed
- Arigo J.T., Eyler D.E., Carroll K.L., Corden J.L. Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol. Cell. 2006;23:841–851. - PubMed
- Bartkova J., Rezaei N., Liontos M., Karakaidos P., Kletsas D., Issaeva N., Vassiliou L.V., Kolettas E., Niforou K., Zoumpourlis V.C. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
Supplemental References
- Lucca, C., Vanoli, F., Cotta-Ramusino, C., Pellicioli, A., Liberi, G., Haber, J., and Foiani, M. (2004). Checkpoint-mediated control of replisome-fork association and signalling in response to replication pausing. Oncogene 23, 1206–1213. - PubMed
- Storici, F., and Resnick, M.A. (2006). The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol. 409, 329–345. - PubMed
- Wach, A., Brachat, A., Pöhlmann, R., and Philippsen, P. (1994). New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous