Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides - PubMed (original) (raw)
Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides
Adrienne Zaprasis et al. Appl Environ Microbiol. 2013 Jan.
Abstract
Bacillus subtilis can attain cellular protection against the detrimental effects of high osmolarity through osmotically induced de novo synthesis and uptake of the compatible solute l-proline. We have now found that B. subtilis can also exploit exogenously provided proline-containing peptides of various lengths and compositions as osmoprotectants. Osmoprotection by these types of peptides is generally dependent on their import via the peptide transport systems (Dpp, Opp, App, and DtpT) operating in B. subtilis and relies on their hydrolysis to liberate proline. The effectiveness with which proline-containing peptides confer osmoprotection varies considerably, and this can be correlated with the amount of the liberated and subsequently accumulated free proline by the osmotically stressed cell. Through gene disruption experiments, growth studies, and the quantification of the intracellular proline pool, we have identified the PapA (YqhT) and PapB (YkvY) peptidases as responsible for the hydrolysis of various types of Xaa-Pro dipeptides and Xaa-Pro-Xaa tripeptides. The PapA and PapB peptidases possess overlapping substrate specificities. In contrast, osmoprotection by peptides of various lengths and compositions with a proline residue positioned at their N terminus was not affected by defects in the PapA and PapB peptidases. Taken together, our data provide new insight into the physiology of the osmotic stress response of B. subtilis. They illustrate the flexibility of this ubiquitously distributed microorganism to effectively exploit environmental resources in its acclimatization to sustained high-osmolarity surroundings through the accumulation of compatible solutes.
Figures
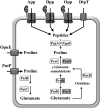
Fig 1
Import, synthesis, and catabolism of
l
-proline in B. subtilis and the generation of free proline through the uptake of proline-containing peptides and their proteolytic breakdown. The osmotically regulated proline transport system OpuE (11, 23), the PutBCP system for the uptake and catabolism of proline (25), and the ABC-type peptide transporters App, Dpp, and Opp (–33, 39) have been described in detail. DtpT is a predicted peptide uptake system (34) and is a member of the POT transporter family (42). Proline biosynthesis in B. subtilis proceeds from the precursor glutamate and involves three enzymes: the γ-glutamate kinase (ProB, ProJ), the γ-glutamyl-phosphate reductase (ProA), and the Δ1-pyrroline-5-carboxylase reductase (ProI, ProH) (28). Anabolic proline biosynthesis is catalyzed by the ProB-ProA-ProI route (marked by white boxes), and osmostress-adaptive proline biosynthesis is catalyzed by the ProJ-ProA-ProH route (marked by gray boxes). In addition to the shown Δ1-pyrroline-5-carboxylase reductases ProI and ProH, an additional protein (ProG) with Δ1-pyrroline-5-carboxylase reductase enzyme activity operates in B. subtilis (28); its physiological role is unclear. The _rocD_-encoded ornithine aminotransferase (RocD) catalyzes an enzymatic reaction that yields the same reaction product as the ProA enzyme and can therefore bypass the joint enzymatic activities of ProB and ProA in proline biosynthesis; a proA rocD double mutant is required to obtain a tight proline auxotrophic growth phenotype of B. subtilis (A. Zaprasis, G. Wünsche, and E. Bremer, unpublished data).
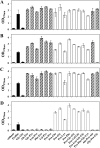
Fig 2
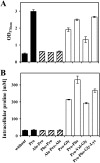
Proline-containing peptides allow growth of proline auxotrophic B. subtilis strains. Cultures of the proline auxotrophs ACB73 (proBA rocD) (A), ACB108 (proBA rocD papA) (B), ACB109 (proBA rocD papB) (C), and ACB97 (proBA rocD papA papB) (D) were grown in SMM in the absence or the presence of the indicated peptides (final concentration in the growth medium, 1 mM) or proline (1 mM). The cells were inoculated to an OD578 of 0.08 from precultures grown in SMM, and the growth yields of the cultures were determined by measuring the OD578 after 16 h of incubation at 37°C. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations. Striped bars indicate those proline-containing peptides whose hydrolysis is dependent on the PapA and PapB peptidases. The App, Dpp, Opp, and DtpT peptide transport systems are intact in all of the B. subtilis strains used in these experiments.
Fig 3
Osmoprotective effects of proline-containing peptides for B. subtilis. Cultures of strain GWB100 (proHJ) (A) and strain GWB19 (proHJ app dpp opp dtpT) (B) were grown in SMM with 1.2 M NaCl in the absence or the presence of the indicated peptides (final concentration, 1 mM, except for the Pro-Pro dipeptide, which was supplied at a concentration of 0.5 mM) and proline (1 mM). The cells were inoculated to an OD578 of 0.1 from precultures grown in SMM. The growth yields of the cultures were determined by measuring the OD578 after incubation for 16 h for strain GWB19 or 20 h for strain GWB100 at 37°C. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations.
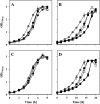
Fig 4
The effect of the putBCP deletion on the growth characteristics of B. subtilis in the presence of various osmoprotective peptides. Cultures were inoculated to optical densities (OD578) of 0.14 from overnight cultures grown in SMM and were incubated at 37°C in SMM (A and C) or SMM with 1.2 M NaCl (B and D) either in the absence (black circles) or in the presence of
l
-proline (gray circles) or the peptides Ala-Pro (black squares), Pro-Gly (white squares), and Gly-Pro (gray squares) (final concentration of proline and of the various peptides was 1 mM). The B. subtilis wild-type strain JH14115 (A and B) and its putBCP mutant derivative, strain ACB221 (C and D), were used in this experiment. Growth of the cultures was monitored over time by measuring the OD578. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations.
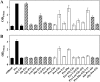
Fig 5
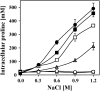
Intracellular proline content of osmotically stressed B. subtilis cells grown in the presence of osmoprotective peptides. Cultures of strain GWB100 (proHJ) were grown in SMM of various salinities either in the absence (white circles) or in the presence of the peptides Ala-Pro (black squares), Pro-Gly (white squares), Gly-Pro (gray squares), and Val-Pro (gray triangles) (final concentration of the various peptides was 1 mM) until they reached an OD578 of 1.8 to 2; the cells were then assayed for their proline content. The proline content of cultures of strain JH14115 (black circles) that was accumulated via de novo synthesis was measured as the control. For each sample analyzed, the intracellular proline content was determined twice. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations. The App, Dpp, Opp, and DtpT peptide transport systems are all intact in the B. subtilis strains GWB100 and JH14115 used for this experiment.
Fig 6
Influence of the PapA and PapB peptidases on osmoprotection by proline-containing peptides and on the intracellular proline pool. (A) Cells of strain ACB118 (proHJ papA papB) were grown in SMM with 1.2 M NaCl in the absence or the presence of the indicated peptides (final concentration, 1 mM) or in the presence of proline (1 mM). The cells were inoculated to an OD578 of 0.1 from a preculture grown in SMM at 37°C. The growth yield of the cultures was measured after 16 h of incubation. (B) The high-osmolarity-grown cells (SMM with 1.2 M NaCl) were harvested once they reached an OD578 of 1.8 to 2 and were then assayed for their proline content. For each sample analyzed, the intracellular proline content was determined twice. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations. Striped bars represent those osmoprotective peptides whose proteolytic breakdown is affected by the PapA and PapB peptidases.
Fig 7
Influence of the catabolic PutBCP system on proline pools formed in response to an external supply of either proline or proline-containing peptides. Cultures of the strain JH14115 (black bars) and its mutant derivatives strain GWB100 (proHJ) (white bars), strain ACB221 (putBCP) (gray bars), and strain ACB223 (proHJ putBCP) (striped bars) were pregrown in SMM and used to inoculate SMM containing 1.2 M NaCl in the absence or presence of
l
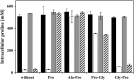
-proline (1 mM) or the peptides Ala-Pro, Pro-Gly, and Gly-Pro (final concentration, 1 mM) to an OD578 of 0.1. The cultures were grown until they reached an OD578 of 1.8 to 2 and were subsequently assayed for their proline content. The values given represent the means of two independently grown cultures, and the error bars indicate standard deviations. For each sample analyzed, the intracellular proline content was determined twice.
Fig 8
The effect of externally provided
l
-proline and osmoprotective peptides on the induction of put expression. The reporter strain ACB225 [ϕ(putB-treA)1] was grown in SMM (A) or in SMM with 1.2 M NaCl (B) to early exponential phase. At time zero, the cells were provided either with
l
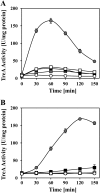
-proline (gray circles) or with the peptide Ala-Pro (black squares), Pro-Gly (white squares), or Gly-Pro (gray squares) (final concentration of proline and of the various peptides was 1 mM). One culture did not receive proline or a peptide (white circles). At the indicated time points, the cells were harvested for TreA reporter enzyme assays. The values for the TreA activity given represent two independently grown cultures, and for each sample analyzed, the TreA activity was determined twice. The error bars indicate standard deviations.
Fig 9
The effect of externally provided proline on growth of different B. subtilis mutant strains. Cultures of the B. subtilis wild type-strain JH642 (A) and its mutant derivatives JSB8 (proHJ) (B), SMB45 (putBCP) (C), and ABB1 (proHJ putBCP) (D) were inoculated to optical densities (OD578) of 0.1 from overnight cultures pregrown in SMM and were incubated at 37°C in SMM (black circles) or in SMM with 1.2 M NaCl either in the absence (white circles) or in the presence of glycine betaine (black triangles), or
l
-proline (gray circles) (final concentrations of glycine betaine and proline were 1 mM). Growth of the cultures was monitored over time by measuring the OD578.
Similar articles
- Uptake of amino acids and their metabolic conversion into the compatible solute proline confers osmoprotection to Bacillus subtilis.
Zaprasis A, Bleisteiner M, Kerres A, Hoffmann T, Bremer E. Zaprasis A, et al. Appl Environ Microbiol. 2015 Jan;81(1):250-9. doi: 10.1128/AEM.02797-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344233 Free PMC article. - Synthesis, release, and recapture of compatible solute proline by osmotically stressed Bacillus subtilis cells.
Hoffmann T, von Blohn C, Stanek A, Moses S, Barzantny H, Bremer E. Hoffmann T, et al. Appl Environ Microbiol. 2012 Aug;78(16):5753-62. doi: 10.1128/AEM.01040-12. Epub 2012 Jun 8. Appl Environ Microbiol. 2012. PMID: 22685134 Free PMC article. - Plant-derived compatible solutes proline betaine and betonicine confer enhanced osmotic and temperature stress tolerance to Bacillus subtilis.
Bashir A, Hoffmann T, Kempf B, Xie X, Smits SHJ, Bremer E. Bashir A, et al. Microbiology (Reading). 2014 Oct;160(Pt 10):2283-2294. doi: 10.1099/mic.0.079665-0. Epub 2014 Jul 10. Microbiology (Reading). 2014. PMID: 25012968 - Guardians in a stressful world: the Opu family of compatible solute transporters from Bacillus subtilis.
Hoffmann T, Bremer E. Hoffmann T, et al. Biol Chem. 2017 Feb 1;398(2):193-214. doi: 10.1515/hsz-2016-0265. Biol Chem. 2017. PMID: 27935846 Review. - Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments.
Kempf B, Bremer E. Kempf B, et al. Arch Microbiol. 1998 Oct;170(5):319-30. doi: 10.1007/s002030050649. Arch Microbiol. 1998. PMID: 9818351 Review.
Cited by
- Sigma Factor Engineering in Actinoplanes sp. SE50/110: Expression of the Alternative Sigma Factor Gene ACSP50_0507 (σHAs) Enhances Acarbose Yield and Alters Cell Morphology.
Schlüter L, Busche T, Bondzio L, Hütten A, Niehaus K, Schneiker-Bekel S, Pühler A, Kalinowski J. Schlüter L, et al. Microorganisms. 2024 Jun 20;12(6):1241. doi: 10.3390/microorganisms12061241. Microorganisms. 2024. PMID: 38930623 Free PMC article. - Uptake of amino acids and their metabolic conversion into the compatible solute proline confers osmoprotection to Bacillus subtilis.
Zaprasis A, Bleisteiner M, Kerres A, Hoffmann T, Bremer E. Zaprasis A, et al. Appl Environ Microbiol. 2015 Jan;81(1):250-9. doi: 10.1128/AEM.02797-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344233 Free PMC article. - Common pathogenic bacteria-induced reprogramming of the host proteinogenic amino acids metabolism.
Li XY, Zeng ZX, Cheng ZX, Wang YL, Yuan LJ, Zhai ZY, Gong W. Li XY, et al. Amino Acids. 2023 Nov;55(11):1487-1499. doi: 10.1007/s00726-023-03334-w. Epub 2023 Oct 9. Amino Acids. 2023. PMID: 37814028 Free PMC article. Review. - Adaptations of Bacillus shacheensis HNA-14 required for long-term survival under osmotic challenge: a multi-omics perspective.
Long X, Tian J, Liao X, Tian Y. Long X, et al. RSC Adv. 2018 Aug 2;8(48):27525-27536. doi: 10.1039/c8ra05472j. eCollection 2018 Jul 30. RSC Adv. 2018. PMID: 35540019 Free PMC article. - Impact of nanoparticles on the Bacillus subtilis (3610) competence.
Eymard-Vernain E, Luche S, Rabilloud T, Lelong C. Eymard-Vernain E, et al. Sci Rep. 2018 Feb 14;8(1):2978. doi: 10.1038/s41598-018-21402-0. Sci Rep. 2018. PMID: 29445231 Free PMC article.
References
- Bremer E, Krämer R. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes, p 79–97 In Storz G, Hengge-Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC
- Booth IR, Edwards MD, Black S, Schumann U, Miller S. 2007. Mechanosensitive channels in bacteria: signs of closure? Nat. Rev. Microbiol. 5:431–440 - PubMed
- Kempf B, Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319–330 - PubMed
- Wood JM, Bremer E, Csonka LN, Krämer R, Poolman B, van der Heide T, Smith LT. 2001. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130:437–460 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases