Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome - PubMed (original) (raw)
doi: 10.1371/journal.pgen.1002945. Epub 2012 Nov 8.
Sarah Walker, Tom Stiff, Emma Hobson, Siripan Limsirichaikul, Gillian Carpenter, Katrina Prescott, Mohnish Suri, Philip J Byrd, Michiko Matsuse, Norisato Mitsutake, Yuka Nakazawa, Pradeep Vasudevan, Margaret Barrow, Grant S Stewart, A Malcolm R Taylor, Mark O'Driscoll, Penny A Jeggo
Affiliations
- PMID: 23144622
- PMCID: PMC3493446
- DOI: 10.1371/journal.pgen.1002945
Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome
Tomoo Ogi et al. PLoS Genet. 2012.
Abstract
A homozygous mutational change in the Ataxia-Telangiectasia and RAD3 related (ATR) gene was previously reported in two related families displaying Seckel Syndrome (SS). Here, we provide the first identification of a Seckel Syndrome patient with mutations in ATRIP, the gene encoding ATR-Interacting Protein (ATRIP), the partner protein of ATR required for ATR stability and recruitment to the site of DNA damage. The patient has compound heterozygous mutations in ATRIP resulting in reduced ATRIP and ATR expression. A nonsense mutational change in one ATRIP allele results in a C-terminal truncated protein, which impairs ATR-ATRIP interaction; the other allele is abnormally spliced. We additionally describe two further unrelated patients native to the UK with the same novel, heterozygous mutations in ATR, which cause dramatically reduced ATR expression. All patient-derived cells showed defective DNA damage responses that can be attributed to impaired ATR-ATRIP function. Seckel Syndrome is characterised by microcephaly and growth delay, features also displayed by several related disorders including Majewski (microcephalic) osteodysplastic primordial dwarfism (MOPD) type II and Meier-Gorlin Syndrome (MGS). The identification of an ATRIP-deficient patient provides a novel genetic defect for Seckel Syndrome. Coupled with the identification of further ATR-deficient patients, our findings allow a spectrum of clinical features that can be ascribed to the ATR-ATRIP deficient sub-class of Seckel Syndrome. ATR-ATRIP patients are characterised by extremely severe microcephaly and growth delay, microtia (small ears), micrognathia (small and receding chin), and dental crowding. While aberrant bone development was mild in the original ATR-SS patient, some of the patients described here display skeletal abnormalities including, in one patient, small patellae, a feature characteristically observed in Meier-Gorlin Syndrome. Collectively, our analysis exposes an overlapping clinical manifestation between the disorders but allows an expanded spectrum of clinical features for ATR-ATRIP Seckel Syndrome to be defined.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
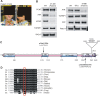
Figure 1. CV1720 cells show impaired ATR–dependent DNA damage responses.
A) WT, DK0064 (ATR–SS), CV1720 (patient), CV1780 (patient's mother) and CV1783 (patient's father) cells were exposed to 5 Jm−2 UV and the mitotic index (MI) assessed 2 h post exposure. A greater than two fold decrease in mitotic index is observed in WT and both paternal cell lines but not in DK0064 (ATR–SS) or CV1720 (patient) cells. B) Cells were exposed to 5 mM HU for 2 h and the percentage of p-H2AX (γ-H2AX) positive cells assessed by immunofluorescence. Note that HU causes pan nuclear p-H2AX formation rather than defined foci as observed after exposure to ionising radiation. Thus, the percentage of γ-H2AX positive cells was scored. C) Cells were exposed to UV (5 Jm−2) and subjected to Western Blotting (WB) using p-Chk1 (p-Ser317) antibodies at 2 h. Chk1 expression was shown to be similar in WT and patient cells (lower panel). D) Cells were exposed to 3 mM HU for 2 h and whole cell extracts analysed by WB using FANCD2 antibodies. The ubiquitylation of FANCD2, detectable by a product with reduced mobility, is diminished in DK0064 (ATR–SS) and CV1720 cells compared to WT cells. E) Cells were exposed to 5 mM HU and examined for the percentage of cells showing >5 53BP1 foci at 2 h post exposure. 53BP1 foci formation is reduced in DK0064 (ATR–SS) and CV1720 cells compared to WT cells. F–I) The indicated cells were processed by WB using ATRIP or ATR antibodies. MCM2 was used as a loading control. F shows the analysis of a range of protein levels for accurate comparison. CV1720 (patient) cells show markedly reduced ATR and ATRIP protein levels. G shows that both parental lines have approximately half the level of ATR and ATRIP compared to two WT cell lines. DK0064 (ATR–SS) and CV1720 cells, in contrast, have more dramatically reduced ATR and ATRIP protein levels. 50 ug protein was loaded. WT in all panels was GM2188. Patient, mother and father were as shown in panel A. H and I show the quantification of ATRIP and ATR protein levels from at least three independent WB experiments.
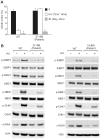
Figure 2. Identification of mutational changes in ATRIP in CV1720.
A) Upper panel shows primer pairs used to distinguish cDNA products encompassing or lacking exon 2. Lower panel shows RT-PCR products obtained using the primers shown in the upper panel. RT-PCR from patient CV1720 generated a smeared product with a defined band of 458 bp, as observed in WT cells, and a weaker band of 325 bp. The latter band was not detected using cDNA from WT cells (MRC5). A similar single 458 bp band was obtained using the same primers with cDNA derived from a distinct wild type cell line (GM2188; data not shown). (B) Sequencing of the RT-PCR products derived from WT (MRC5) and patient (CV1720) cells. A double sequence pattern at the exon 2–3 boundary is observed using patient CV1720 cDNA. C) Selective quantitative amplification of the WT or 2278C>T ATRIP alleles. Primers located in ATRIP exon 12 and 13 were designed to selectively amplify the WT (c.2278C) (P1 and P3C) versus the mutated (c.2278C>T) (P2 and P3C) alleles. The WT PCR product is shown in blue and the c.2278C>T PCR product in red. The exon 12 mutated allele is only observed in the patient and mother cDNA whilst the WT allele is observed in the patient, mother and father cDNA although the level is reduced in the patient and mother. D) qRT-PCR analysis of ATRIP splicing variants from patient CV1720 and parental cells. qRT-PCR analysis of the level of the normally spliced (encompassing exons 1-2-3) and the aberrantly spliced (Δexon2) ATRIP cDNA in the patient and parent cells. PCR primers were designed at the exon2-exon3 or exon1-exon3 boundaries to selectively amplify the splicing variants. Transcripts from HPRT1 were used as a quantification control. The correctly spliced transcript from the paternal allele of the patient (wild type c.2278C, blue fraction in the cumulative bar labelled, ‘patient’, at the left panel) was estimated to be ∼25% of the normal level. (E) The mis-spliced paternal allele is subject to nonsense mediated mRNA decay (NMD). Cycleave-qPCR confirmed that the ATRIP c.2278C>T mutant allele was expressed exclusively in the patient and the mother. The ATRIP exon12-13 fragment was amplified with PCR primers P7/P8 as shown in the figure. A set of fluorescent probes were used to distinguish the WT versus c.2278C>T allele (probe1 and probe2, respectively). In the patient, the paternal mRNA transcript level (emerald lines) is low because of NMD (top left). Puromycin treatment eliminated the NMD and the paternal transcript level returned to the normal level. In all panels WT represented MRC5, patient was CV1720 and parents were as shown in Figure 1A.
Figure 3. WT ATRIP cDNA but not cDNA encoding p.Arg760* ATRIP complements the G2/M checkpoint defect in CV1720 cells, and p.Arg760*ATRIP impairs ATR–ATRIP protein interaction.
A) Analysis of the G2/M checkpoint defect in CV1720 cells following expression of ATRIP cDNA. G2/M checkpoint arrest was examined 2 h post exposure to 5 Jm−2 UV. As shown in Figure 1A, WT cells showed proficient checkpoint arrest whilst DK0064 (ATR–SS) and CV1720 (patient) cells are unable to undergo arrest. Expression of WT ATRIP cDNA restored the ability of CV1720 (patient) and DK0064 (ATR–SS) to undergo checkpoint arrest but this was not observed following transfection of cDNA encoding R760* ATRIP. Significantly, expression of ATRIP R760* did not impair checkpoint arrest in WT cells verifying that it does not exert a dominant negative impact. Results represent the mean and SD of three experiments. WT cells were GM2188. ATR–SS represents DK0064 and patient, CV1720. B) R760* ATRIP impairs ATR–ATRIP interaction. Crude lysates were prepared from HEK293T cells and either mock transfected (lane1), transfected with HA-tagged WT ATRIP cDNA (lane2), or R760* ATRIP cDNA (lane3) (generating p.Arg760* ATRIP protein) together with ATR cDNA. The extracts were immunoprecipitated with agarose-conjugated rabbit anti-HA-tag antibody (MBL). Interaction with ATR was examined by immunoblotting with ATR antibodies (left panel). Immunoblotting using the HA-tag (ATRIP; right panel) verified expression of the appropriately sized ATRIP in the samples. 33% of the crude lysate was loaded; IP, immunoprecipitate.
Figure 4. Patients 27-4BI and 19-8BI have reduced ATR and ATRIP expression and mutations in ATR.
A) Photographs of patient included with informed consent of parent. B) Cell extracts (50 µg) from LBLs derived from WT (IM257), patient 27-4BI or patient 19-8BI were immunoblotted using the indicated antibodies. Reduced expression of ATR was observed in both patients. 27-4BI also had reduced ATRIP expression. C) Structure of ATR showing the site of the mutations identified and the UME domain. D) The UME domain is conserved between species and the methionine residue within this domain is conserved in yeast.
Figure 5. LBLs from patient 27-4BI and 19-8BI showed impaired ATR–dependent damage responses.
A) 27-4BI cells were examined for their ability to activate G2/M checkpoint arrest at 4 h following exposure to 7 Jm−2 UV. In contrast to WT cells (GM2188), no significant arrest was observed in 27-4BI cells. The checkpoint response to ionizing radiation, which is ATM rather than ATR dependent, was normal. B) LBLs derived from patients 27-4BI and 19-8BI were examined for their ability to phosphorylate the indicated ATR substrates at 1 h following exposure to 0.5 mM HU. WT represents IM257. 27-4BI and control LBLs have a similar cell cycle profile demonstrating that the lack of ATR substrate phosphorylation cannot be attributed to the lack of S phase cells (Figure S2).
Similar articles
- Deficiency in origin licensing proteins impairs cilia formation: implications for the aetiology of Meier-Gorlin syndrome.
Stiff T, Alagoz M, Alcantara D, Outwin E, Brunner HG, Bongers EM, O'Driscoll M, Jeggo PA. Stiff T, et al. PLoS Genet. 2013;9(3):e1003360. doi: 10.1371/journal.pgen.1003360. Epub 2013 Mar 14. PLoS Genet. 2013. PMID: 23516378 Free PMC article. - Replication stress, microcephalic primordial dwarfism, and compromised immunity in ATRIP deficient patients.
Duthoo E, Beyls E, Backers L, Gudjónsson T, Huang P, Jonckheere L, Riemann S, Parton B, Du L, Debacker V, De Bruyne M, Hoste L, Baeyens A, Vral A, Van Braeckel E, Staal J, Mortier G, Kerre T, Pan-Hammarström Q, Sørensen CS, Haerynck F, Claes KBM, Tavernier SJ. Duthoo E, et al. J Exp Med. 2025 May 5;222(5):e20241432. doi: 10.1084/jem.20241432. Epub 2025 Mar 3. J Exp Med. 2025. PMID: 40029331 - CtIP Mutations Cause Seckel and Jawad Syndromes.
Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, Jackson SP, Børglum AD. Qvist P, et al. PLoS Genet. 2011 Oct;7(10):e1002310. doi: 10.1371/journal.pgen.1002310. Epub 2011 Oct 6. PLoS Genet. 2011. PMID: 21998596 Free PMC article. - An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome.
O'Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. O'Driscoll M, et al. DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1227-35. doi: 10.1016/j.dnarep.2004.03.025. DNA Repair (Amst). 2004. PMID: 15279811 Review.
Cited by
- Disease-associated DNA2 nuclease-helicase protects cells from lethal chromosome under-replication.
Falquet B, Ölmezer G, Enkner F, Klein D, Challa K, Appanah R, Gasser SM, Rass U. Falquet B, et al. Nucleic Acids Res. 2020 Jul 27;48(13):7265-7278. doi: 10.1093/nar/gkaa524. Nucleic Acids Res. 2020. PMID: 32544229 Free PMC article. - Targeting ATR in patients with cancer.
Ngoi NYL, Pilié PG, McGrail DJ, Zimmermann M, Schlacher K, Yap TA. Ngoi NYL, et al. Nat Rev Clin Oncol. 2024 Apr;21(4):278-293. doi: 10.1038/s41571-024-00863-5. Epub 2024 Feb 20. Nat Rev Clin Oncol. 2024. PMID: 38378898 Review. - Genetics of the patella.
Samuels ME, Campeau PM. Samuels ME, et al. Eur J Hum Genet. 2019 May;27(5):671-680. doi: 10.1038/s41431-018-0329-6. Epub 2019 Jan 21. Eur J Hum Genet. 2019. PMID: 30664715 Free PMC article. Review. - Primordial dwarfism: overview of clinical and genetic aspects.
Khetarpal P, Das S, Panigrahi I, Munshi A. Khetarpal P, et al. Mol Genet Genomics. 2016 Feb;291(1):1-15. doi: 10.1007/s00438-015-1110-y. Epub 2015 Sep 1. Mol Genet Genomics. 2016. PMID: 26323792 Review. - Microcephalic Osteodysplastic Primordial Dwarfism, Type II: a Clinical Review.
Bober MB, Jackson AP. Bober MB, et al. Curr Osteoporos Rep. 2017 Apr;15(2):61-69. doi: 10.1007/s11914-017-0348-1. Curr Osteoporos Rep. 2017. PMID: 28409412 Free PMC article. Review.
References
- Majewski F, Goecke T (1982) Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. Am J Med Genet 12: 7–21. - PubMed
- Hall JG, Flora C, Scott CI Jr, Pauli RM, Tanaka KI (2004) Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am J Med Genet A 130: 55–72. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous