Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells - PubMed (original) (raw)
Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells
Renyue Bao et al. PLoS One. 2012.
Abstract
Constitutive Wnt signalling is characterized by excessive levels of β-catenin protein and is a frequent occurrence in cancer. APC and Axin are key components of the β-catenin destruction complex that acts to promote β-catenin degradation. The levels of Axin are in turn controlled by tankyrases, members of the PARP-family of poly-ADP-ribosylation enzymes. In colorectal cancer cells, which typically harbor APC mutations, inhibition of tankyrase activity promotes Axin stabilization and attenuates Wnt signalling. Here, we examined the effect of inhibiting tankyrases in breast cancer cells with normal APC. We show that application of the small molecule tankyrase inhibitor, XAV939 or siRNA-mediated abrogation of tankyrase expression increases Axin1 and Axin2 protein levels and attenuates Wnt-induced transcriptional responses in several breast cancer lines. In MDA-MB-231 cells, inhibiton of tankyrase activity also attenuate Wnt3a induced cell migration. Moreover, in both MDA-MB-231 and colorectal cancer cells, XAV939 inhibits cell growth under conditions of serum-deprivation. However, the presence of serum prevents this growth inhibitory effect, although inhibition of Wnt-induced transcriptional and migratory responses was maintained. These results indicate that stabilization of Axin by inhibition of tankyrases alone, may not be an effective means to block tumor cell growth and that combinatorial therapeutic approaches should be considered.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
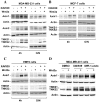
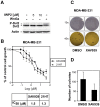
Figure 1. XAV939, a chemical inhibitor of tankyrases, stabilizes Axin in breast cancer cells.
(A–C) Human MDA-MB-231 and MCF-7 cells and mouse EMT6 cells were treated overnight with 10 µM XAV939 in the presence or in the absence of Wnt3a conditioned medium either overnight or for 4 h and protein levels of Axin1, Axin2 and tankyrases (TNKS) were determined by immunoblotting of total cell lysates. (D) MDA-MB-231 cells were treated overnight with 10 µM XAV939 and IWR-1-endo and after 4 h incubation with Wnt3a conditioned medium, cells were lysed and subjected to immunoblot analysis using anti-Axin1, anti-Axin2 and anti-Tankyrase antibodies. All samples were also probed with anti-actin antibody to verify equal protein loading.
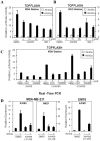
Figure 2. XAV939 blocks Wnt/β-catenin signalling in breast cancer cells.
(A–B) Tankyrase inhibitors, XAV939 and IWR-1-endo, attenuate TOPFLASH activity in breast cancer MDA-MB-231 cells and in colon cancer RKO cells. MDA-MB-231 and RKO cells, stably expressing the Wnt-responsive TOPFLASH/pBAR and the control Renilla Luciferase reporter were treated with XAV939 and IWR-1-endo at the indicated concentrations in serum depleted medium (0.2% FBS) followed by 4 h incubation with control or Wnt3a conditioned medium. Cells were lysed and luciferase activities were measured. Data represents the mean of three replicates +/− standard deviation. (C) Inhibition of TOPFLASH activity by XAV939 in MDA-MB-231 stable cells maintained in medium supplemented with different FBS concentrations. Cells were treated overnight with 5 µM 21H7 and 1 or 5 µM XAV939 in the presence of control or Wnt3a conditioned medium with 5%, 1.25% or 0.2% FBS. Promoter activity was measured by luciferase assay. Data is shown as the mean of three replicates +/− standard deviation. (D) XAV939 decreases expression of Wnt target genes. MDA-MB-231 and EMT6 cells were treated with 10 μM XAV939 and Wnt3a-conditioned media overnight and the expression of Wnt target genes AXIN2 and NKD1 was measured by Real-Time PCR. Relative gene expression is plotted as the average of three PCR replicates +/− the range.
Figure 3. Knockdown of tankyrases inhibits Wnt/β-catenin signalling.
(A) MDA-MB-231 and RKO stable cells were transfected with 20 nM siControl (siCTL) or siTankyrases 1 (TNKS1), siTankyrases 2 (TNKS2) or both. After 48 h, cells were cultured in the presence of control or Wnt3a conditioned medium for 4 h and were lysed. TOPFLASH activity was measured by luciferase assay and Firefly luciferase activity was normalized to Renilla luciferase activity, used as internal control. Values are shown as the mean of three replicates +/− standard deviations. (B) RNA was isolated from MDA-MB-231 cells, transfected with siCTL, siTankyrase 1, siTankyrase 2 or siTankyrase 1/2, and was subjected to Real Time PCR analysis to confirm knockdown efficiency. Relative gene expression is plotted as the average of three PCR replicates +/− the range.
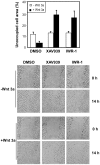
Figure 4. XAV939 inhibits migration of MDA-MB-231 cells.
(A) A wound was introduced to confluent MDA-MB-231 monolayers and cells were then treated with 5 µM XAV939, 5 µM IWR-1 or DMSO in the presence of control or Wnt3a conditioned medium, supplemented with 2.5% FBS. A plot of the cell-free area within the wound, determined using Image J, is presented as percentage of the concurrent control at 0 h. (B) Representative microphotographs at 0 h and 14 h after wounding are shown.
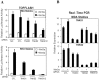
Figure 5. XAV939 suppresses growth and colony formation in MDA-MB-231 cells in low serum conditions.
(A) MDA-MB-231 cells were treated overnight with the indicated concentrations of IWP-2 or with Wnt3a conditioned medium for 24h. Cell lysates were subjected to immunoblotting with Dvl2 antibody. Equal protein loading was verified by immunoblotting with anti-actin antibody. (B) MDA-MB-231 cells were treated for 72 h with the indicated concentrations of XAV939 or 21H7 in medium with low serum (1.25% FBS) and cell growth was determined by SRB assay. IC50 values for growth inhibition by XAV939 and 21H7 were calculated using GraphPad Prism 5.0 software and are shown (lower panel). (C and D) XAV939 impairs cell colony formation in MDA-MB-231 cells grown in low serum (1.25% FBS) conditions. Representative photographs are shown (C) Colony numbers were counted and a plot showing the average of 2 independent experiments +/− standard deviations is shown (D).
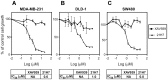
Figure 6. XAV939 has no effect on growth of cancer cells in optimal growth conditions.
Growth inhibition was determined in human breast cancer cells MDA-MB-231 (A) and in human colon cancer cells DLD-1 (B) and SW480 (C) following treatment with XAV939 or 21H7 for 72 h. Cells were maintained in media with optimal serum concentration (5% FBS for MDA-MB-231 cells and 10% FBS for DLD-1 and SW480 cells). Under these conditions 21H7 potently decreases growth of breast and colon cancer cells whereas XAV939 has no effect. Graphs represent mean values +/− standard deviations from three independent experiments done in triplicate. IC50 values for growth inhibition (where applicable) are shown.
Similar articles
- Tankyrase inhibitors attenuate WNT/β-catenin signaling and inhibit growth of hepatocellular carcinoma cells.
Ma L, Wang X, Jia T, Wei W, Chua MS, So S. Ma L, et al. Oncotarget. 2015 Sep 22;6(28):25390-401. doi: 10.18632/oncotarget.4455. Oncotarget. 2015. PMID: 26246473 Free PMC article. - A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth.
Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, Machon O, Korinek V, Choo E, Diaz D, Merchant M, Polakis P, Holsworth DD, Krauss S, Costa M. Lau T, et al. Cancer Res. 2013 May 15;73(10):3132-44. doi: 10.1158/0008-5472.CAN-12-4562. Epub 2013 Mar 28. Cancer Res. 2013. PMID: 23539443 - APC Mutations as a Potential Biomarker for Sensitivity to Tankyrase Inhibitors in Colorectal Cancer.
Tanaka N, Mashima T, Mizutani A, Sato A, Aoyama A, Gong B, Yoshida H, Muramatsu Y, Nakata K, Matsuura M, Katayama R, Nagayama S, Fujita N, Sugimoto Y, Seimiya H. Tanaka N, et al. Mol Cancer Ther. 2017 Apr;16(4):752-762. doi: 10.1158/1535-7163.MCT-16-0578. Epub 2017 Feb 8. Mol Cancer Ther. 2017. PMID: 28179481 - Regulation of Wnt/β-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding.
Mariotti L, Pollock K, Guettler S. Mariotti L, et al. Br J Pharmacol. 2017 Dec;174(24):4611-4636. doi: 10.1111/bph.14038. Epub 2017 Nov 5. Br J Pharmacol. 2017. PMID: 28910490 Free PMC article. Review. - Targeting Tankyrase to Fight WNT-dependent Tumours.
Thorvaldsen TE. Thorvaldsen TE. Basic Clin Pharmacol Toxicol. 2017 Aug;121(2):81-88. doi: 10.1111/bcpt.12786. Epub 2017 May 10. Basic Clin Pharmacol Toxicol. 2017. PMID: 28371398 Review.
Cited by
- β-catenin mediates growth defects induced by centrosome loss in a subset of APC mutant colorectal cancer independently of p53.
Bourmoum M, Radulovich N, Sharma A, Tkach JM, Tsao MS, Pelletier L. Bourmoum M, et al. PLoS One. 2024 Feb 7;19(2):e0295030. doi: 10.1371/journal.pone.0295030. eCollection 2024. PLoS One. 2024. PMID: 38324534 Free PMC article. - The Poly(ADP-ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-ribosylation of Axin and APC2.
Croy HE, Fuller CN, Giannotti J, Robinson P, Foley AVA, Yamulla RJ, Cosgriff S, Greaves BD, von Kleeck RA, An HH, Powers CM, Tran JK, Tocker AM, Jacob KD, Davis BK, Roberts DM. Croy HE, et al. J Biol Chem. 2016 Jun 10;291(24):12747-12760. doi: 10.1074/jbc.M115.705442. Epub 2016 Apr 11. J Biol Chem. 2016. PMID: 27068743 Free PMC article. - New insights into the regulation of Axin function in canonical Wnt signaling pathway.
Song X, Wang S, Li L. Song X, et al. Protein Cell. 2014 Mar;5(3):186-93. doi: 10.1007/s13238-014-0019-2. Epub 2014 Jan 29. Protein Cell. 2014. PMID: 24474204 Free PMC article. Review. - Genome-Wide Meta-Analysis Identifies Two Novel Risk Loci for Epilepsy.
Song M, Liu J, Yang Y, Lv L, Li W, Luo XJ. Song M, et al. Front Neurosci. 2021 Aug 12;15:722592. doi: 10.3389/fnins.2021.722592. eCollection 2021. Front Neurosci. 2021. PMID: 34456681 Free PMC article. - Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment.
Vilchez V, Turcios L, Marti F, Gedaly R. Vilchez V, et al. World J Gastroenterol. 2016 Jan 14;22(2):823-32. doi: 10.3748/wjg.v22.i2.823. World J Gastroenterol. 2016. PMID: 26811628 Free PMC article. Review.
References
- Clevers H, Nusse R (2012) Wnt/beta-catenin signaling and disease. Cell 149: 1192–1205. - PubMed
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, et al. (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512. - PubMed
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous