Heparin oligosaccharides inhibit chemokine (CXC motif) ligand 12 (CXCL12) cardioprotection by binding orthogonal to the dimerization interface, promoting oligomerization, and competing with the chemokine (CXC motif) receptor 4 (CXCR4) N terminus - PubMed (original) (raw)
Heparin oligosaccharides inhibit chemokine (CXC motif) ligand 12 (CXCL12) cardioprotection by binding orthogonal to the dimerization interface, promoting oligomerization, and competing with the chemokine (CXC motif) receptor 4 (CXCR4) N terminus
Joshua J Ziarek et al. J Biol Chem. 2013.
Abstract
The ability to interact with cell surface glycosaminoglycans (GAGs) is essential to the cell migration properties of chemokines, but association with soluble GAGs induces the oligomerization of most chemokines including CXCL12. Monomeric CXCL12, but not dimeric CXCL12, is cardioprotective in a number of experimental models of cardiac ischemia. We found that co-administration of heparin, a common treatment for myocardial infarction, abrogated the protective effect of CXCL12 in an ex vivo rat heart model for myocardial infarction. The interaction between CXCL12 and heparin oligosaccharides has previously been analyzed through mutagenesis, in vitro binding assays, and molecular modeling. However, complications from heparin-induced CXCL12 oligomerization and studies using very short oligosaccharides have led to inconsistent conclusions as to the residues involved, the orientation of the binding site, and whether it overlaps with the CXCR4 N-terminal site. We used a constitutively dimeric variant to simplify the NMR analysis of CXCL12-binding heparin oligosaccharides of varying length. Biophysical and mutagenic analyses reveal a CXCL12/heparin interaction surface that lies perpendicular to the dimer interface, does not involve the chemokine N terminus, and partially overlaps with the CXCR4-binding site. We further demonstrate that heparin-mediated enzymatic protection results from the promotion of dimerization rather than direct heparin binding to the CXCL12 N terminus. These results clarify the structural basis for GAG recognition by CXCL12 and lend insight into the development of CXCL12-based therapeutics.
Figures
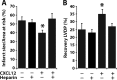
FIGURE 1.
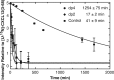
Heparin eliminates CXCL12-mediated cardioprotection. Isolated rat hearts (n = 4) were buffer-perfused for 15 min, followed by aerobic perfusion with either CXCL12 (50 n
m
) or CXCL12 (50 n
m
) in combination with medicinal heparin (50 units ml−1) for an additional 15 min. The hearts were subjected to 30 min of no-flow global ischemia followed by 180 min of reperfusion. The percentage of infarct size and area at risk (A) and recovery left ventricle diastolic pressure (LVDP) (B) were measured in the left ventricle relative to nonischemic controls. The asterisk indicates statistical significance as determined by Student's t test. A, p = 0.0006. B, p = 0.006.
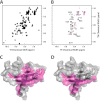
FIGURE 2.
Heparin dodecasaccharide produces extensive CXCL12 line broadening. A and B, 50 μ
m
15N-CXCL12 in the absence (A) and presence (B) of 150 μ
m
dp12. Exchange broadening eliminated most signals at 0.5 equivalent of dp12. C, residues that exhibited extensive line broadening are colored magenta on the surface of CXCL12 (Protein Data Bank code 2KEE); broadening was observed in residues 15, 17–20, 23–29, 31, 37–42, 46, 48–51, 54–55, 58–63, 66, and 67. D, residues located at the dimer interface in the CXCL12 crystal structure (Protein Data Bank code 2J7Z) highlighted in magenta on the surface of CXCL12 (Protein Data Bank code 2KEE).
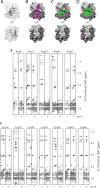
FIGURE 3.
Heparin binds orthogonal to the CXCL12 dimer interface in a size-dependent manner. A, CXCL122 ribbon and surface representation in the absence of heparin; the two protomers are shaded in light and dark gray (N-terminal residues 1–8 are absent for ease of viewing). B–D, CXCL12 surface illustration in the presence of 3 equivalents of dp4 (B), dp8 (C), and dp12 (D). Residues that experience substantial chemical shift perturbations (magenta) and complete line-broadening (green) are mapped onto the CXCL122 NMR structure (Protein Data Bank code ID 2K01). E and F, HCCH-TOCSY Hα strips of all CXCL122 arginine (E) and lysine (F) residues in the absence and presence of heparin decasaccharide. Side chain correlations are labeled, and a dashed line indicates the positions of apo-CXCL122 resonances. Strong resonances near the Arg-47 Hδ peak in the presence of heparin are signals from Arg-8 that bleed through from a neighboring plane of the three-dimensional spectrum.
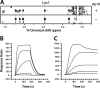
FIGURE 4.
The CXCR41–38 and heparin-binding sites overlap. A, addition of CXCR41–38 peptide fails to disrupt the CXCL122-dp4 complex. Spectra for 50 μ
m
CXCL122 (black), 50 μ
m
CXCL122 + 150 μ
m
dp4 (orange), and 50 μ
m
CXCL122 + 150 μ
m
dp4 + 200 μ
m
CXCR41–38 (blue) are overlaid. B, close-up views of HSQC signals highlighted in A, showing that peaks shift upon dp4 binding but are unperturbed by the addition of CXCR41–38 peptide. C, 50 n
m
CXCL122 in the presence (dashed line) or absence (solid line) of 500 n
m
CXCR41–38 was injected over a heparin chip at a flow rate of 30 μl min−1. An increase in response units is indicative of binding to the heparin chip.
FIGURE 5.
Heparin does not interact with the CXCL122 N termini. A, HCCH-TOCSY Hα strip for Lys-1 of CXCL122 in the presence (top panel) and absence (bottom panel) of heparin decasaccharide. Side chain correlations are labeled, and a dashed line indicates the positions of apo-CXCL122 resonances. B and C, SPR sensorgrams of 8, 16, 32, 63, and 125 n
m
CXCL12(3–68) (B) and CXCL122(3–68) (C) injected over a heparin chip at a flow rate of 30 μl min−1. An increase in response units is indicative of binding to the heparin chip.
FIGURE 6.
Heparin protects the CXCL12 N terminus from CD26/DPPIV degradation by promoting dimerization. 10 μ
m
CXCL12 and 2 μ
m
[_U_-15N]CXCL12(3–68) was incubated with 0.2 ng μl−1 DPPIV/CD26 in the absence (squares) or presence of 36 μ
m
dp2 (diamonds) or dp4 (circles). At the indicated time points, samples were desalted, mixed with sinapinic acid, and spotted onto a MALDI-TOF plate. The intensity of CXCL12(3–68) product formation was normalized to the [_U_-15N]CXCL12(3–68) internal standard. The intensities at each time point were subtracted from 5, the maximum ratio of unlabeled to labeled CXCL12(3–68), and fitted to the half-life equation.
FIGURE 7.
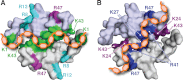
Schematic representation of the heparin-CXCL12 binding mode. A, the previously published binding mode proposed that heparin (orange) associates with CXL12 (gray) along the dimerization interface, primarily contacting the β1 strands and the Lys-1 residue of each protomer. Residues highlighted on the CXCL12 surface were identified in previous studies by Lortat-Jacob and co-workers (–29) (green), Lolis and co-workers (30) (magenta), and Laguri et al. (31) (cyan). B, our combined biophysical analyses support a new model in which heparin promotes CXCL12 dimerization by contacting residues along the entire six-stranded sheet. The highlighted CXCL12 residues associate with heparin as determined by two-dimensional NMR, mutagenesis, SPR (blue), and three-dimensional NMR (purple).
Similar articles
- Structural and functional basis of CXCL12 (stromal cell-derived factor-1 alpha) binding to heparin.
Murphy JW, Cho Y, Sachpatzidis A, Fan C, Hodsdon ME, Lolis E. Murphy JW, et al. J Biol Chem. 2007 Mar 30;282(13):10018-10027. doi: 10.1074/jbc.M608796200. Epub 2007 Jan 29. J Biol Chem. 2007. PMID: 17264079 Free PMC article. - Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis.
Fermas S, Gonnet F, Sutton A, Charnaux N, Mulloy B, Du Y, Baleux F, Daniel R. Fermas S, et al. Glycobiology. 2008 Dec;18(12):1054-64. doi: 10.1093/glycob/cwn088. Epub 2008 Sep 16. Glycobiology. 2008. PMID: 18796646 - Solution NMR characterization of chemokine CXCL8/IL-8 monomer and dimer binding to glycosaminoglycans: structural plasticity mediates differential binding interactions.
Joseph PR, Mosier PD, Desai UR, Rajarathnam K. Joseph PR, et al. Biochem J. 2015 Nov 15;472(1):121-33. doi: 10.1042/BJ20150059. Epub 2015 Sep 14. Biochem J. 2015. PMID: 26371375 Free PMC article. - CXCL14 antagonizes the CXCL12-CXCR4 signaling axis.
Hara T, Tanegashima K. Hara T, et al. Biomol Concepts. 2014 May;5(2):167-73. doi: 10.1515/bmc-2014-0007. Biomol Concepts. 2014. PMID: 25372750 Review. - Role of the SDF-1/CXCR4 system in myocardial infarction.
Takahashi M. Takahashi M. Circ J. 2010 Mar;74(3):418-23. doi: 10.1253/circj.cj-09-1021. Epub 2010 Jan 30. Circ J. 2010. PMID: 20118565 Review.
Cited by
- CXCL12 chemokine dimer signaling modulates acute myelogenous leukemia cell migration through altered receptor internalization.
Drouillard D, Halyko M, Cinquegrani E, McAllister D, Peterson FC, Marchese A, Dwinell MB. Drouillard D, et al. bioRxiv [Preprint]. 2024 Aug 27:2024.08.26.609725. doi: 10.1101/2024.08.26.609725. bioRxiv. 2024. PMID: 39253415 Free PMC article. Preprint. - The acidic intrinsically disordered region of the inflammatory mediator HMGB1 mediates fuzzy interactions with CXCL12.
Mantonico MV, De Leo F, Quilici G, Colley LS, De Marchis F, Crippa M, Mezzapelle R, Schulte T, Zucchelli C, Pastorello C, Carmeno C, Caprioglio F, Ricagno S, Giachin G, Ghitti M, Bianchi ME, Musco G. Mantonico MV, et al. Nat Commun. 2024 Feb 8;15(1):1201. doi: 10.1038/s41467-024-45505-7. Nat Commun. 2024. PMID: 38331917 Free PMC article. - Chemokine Heteromers and Their Impact on Cellular Function-A Conceptual Framework.
Blanchet X, Weber C, von Hundelshausen P. Blanchet X, et al. Int J Mol Sci. 2023 Jun 30;24(13):10925. doi: 10.3390/ijms241310925. Int J Mol Sci. 2023. PMID: 37446102 Free PMC article. Review. - Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads.
Adrian E, Treľová D, Filová E, Kumorek M, Lobaz V, Poreba R, Janoušková O, Pop-Georgievski O, Lacík I, Kubies D. Adrian E, et al. Int J Mol Sci. 2021 Oct 28;22(21):11666. doi: 10.3390/ijms222111666. Int J Mol Sci. 2021. PMID: 34769095 Free PMC article. - Combination of dociparstat sodium (DSTAT), a CXCL12/CXCR4 inhibitor, with azacitidine for the treatment of hypomethylating agent refractory AML and MDS.
Huselton E, Rettig MP, Campbell K, Cashen AF, DiPersio JF, Gao F, Jacoby MA, Pusic I, Romee R, Schroeder MA, Uy GL, Marcus S, Westervelt P. Huselton E, et al. Leuk Res. 2021 Nov;110:106713. doi: 10.1016/j.leukres.2021.106713. Epub 2021 Sep 22. Leuk Res. 2021. PMID: 34619434 Free PMC article. Clinical Trial.
References
- Fuster V., Hurst J. W. (2004) Hurst's the Heart, 11th Ed., McGraw-Hill, Medical Publishing Division, New York
- Antman E. M., Anbe D. T., Armstrong P. W., Bates E. R., Green L. A., Hand M., Hochman J. S., Krumholz H. M., Kushner F. G., Lamas G. A., Mullany C. J., Ornato J. P., Pearle D. L., Sloan M. A., Smith S. C., Jr., Alpert J. S., Anderson J. L., Faxon D. P., Fuster V., Gibbons R. J., Gregoratos G., Halperin J. L., Hiratzka L. F., Hunt S. A., Jacobs A. K. (2004) ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 110, 588–636 - PubMed
- Rosenberg R. D., Damus P. S. (1973) The purification and mechanism of action of human antithrombin-heparin cofactor. J. Biol. Chem. 248, 6490–6505 - PubMed
- Handel T. M., Johnson Z., Crown S. E., Lau E. K., Proudfoot A. E. (2005) Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu. Rev. Biochem. 74, 385–410 - PubMed
- Johnson Z., Proudfoot A. E., Handel T. M. (2005) Interaction of chemokines and glycosaminoglycans. A new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 16, 625–636 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM097381/GM/NIGMS NIH HHS/United States
- R56 AI063325/AI/NIAID NIH HHS/United States
- R01 AI080363/AI/NIAID NIH HHS/United States
- GM097381/GM/NIGMS NIH HHS/United States
- R01 HL062244/HL/NHLBI NIH HHS/United States
- R01 HL054075/HL/NHLBI NIH HHS/United States
- HL62244/HL/NHLBI NIH HHS/United States
- R01 AI063325/AI/NIAID NIH HHS/United States
- R15 CA159202/CA/NCI NIH HHS/United States
- GM38060/GM/NIGMS NIH HHS/United States
- R37 AI058072/AI/NIAID NIH HHS/United States
- AI080363/AI/NIAID NIH HHS/United States
- AI058072/AI/NIAID NIH HHS/United States
- 1-R15CA159202-01/CA/NCI NIH HHS/United States
- R01 AI058072/AI/NIAID NIH HHS/United States
- R01 GM038060/GM/NIGMS NIH HHS/United States
- AI063325/AI/NIAID NIH HHS/United States
- HL54075/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources