Variant of TREM2 associated with the risk of Alzheimer's disease - PubMed (original) (raw)
. 2013 Jan 10;368(2):107-16.
doi: 10.1056/NEJMoa1211103. Epub 2012 Nov 14.
Hreinn Stefansson, Stacy Steinberg, Ingileif Jonsdottir, Palmi V Jonsson, Jon Snaedal, Sigurbjorn Bjornsson, Johanna Huttenlocher, Allan I Levey, James J Lah, Dan Rujescu, Harald Hampel, Ina Giegling, Ole A Andreassen, Knut Engedal, Ingun Ulstein, Srdjan Djurovic, Carla Ibrahim-Verbaas, Albert Hofman, M Arfan Ikram, Cornelia M van Duijn, Unnur Thorsteinsdottir, Augustine Kong, Kari Stefansson
Affiliations
- PMID: 23150908
- PMCID: PMC3677583
- DOI: 10.1056/NEJMoa1211103
Variant of TREM2 associated with the risk of Alzheimer's disease
Thorlakur Jonsson et al. N Engl J Med. 2013.
Abstract
Background: Sequence variants, including the ε4 allele of apolipoprotein E, have been associated with the risk of the common late-onset form of Alzheimer's disease. Few rare variants affecting the risk of late-onset Alzheimer's disease have been found.
Methods: We obtained the genome sequences of 2261 Icelanders and identified sequence variants that were likely to affect protein function. We imputed these variants into the genomes of patients with Alzheimer's disease and control participants and then tested for an association with Alzheimer's disease. We performed replication tests using case-control series from the United States, Norway, The Netherlands, and Germany. We also tested for a genetic association with cognitive function in a population of unaffected elderly persons.
Results: A rare missense mutation (rs75932628-T) in the gene encoding the triggering receptor expressed on myeloid cells 2 (TREM2), which was predicted to result in an R47H substitution, was found to confer a significant risk of Alzheimer's disease in Iceland (odds ratio, 2.92; 95% confidence interval [CI], 2.09 to 4.09; P=3.42×10(-10)). The mutation had a frequency of 0.46% in controls 85 years of age or older. We observed the association in additional sample sets (odds ratio, 2.90; 95% CI, 2.16 to 3.91; P=2.1×10(-12) in combined discovery and replication samples). We also found that carriers of rs75932628-T between the ages of 80 and 100 years without Alzheimer's disease had poorer cognitive function than noncarriers (P=0.003).
Conclusions: Our findings strongly implicate variant TREM2 in the pathogenesis of Alzheimer's disease. Given the reported antiinflammatory role of TREM2 in the brain, the R47H substitution may lead to an increased predisposition to Alzheimer's disease through impaired containment of inflammatory processes. (Funded by the National Institute on Aging and others.).
Figures
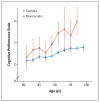
Figure 1. Cognition as a Function of Age in Controls Who Were Carriers or Noncarriers of the rs75932628-T Variant Associated with the Risk of Alzheimer’s Disease
Shown are scores on the Cognitive Performance Scale (CPS) for carriers and noncarriers of the rs75932628-T variant associated with Alzheimer’s disease, according to age. Scores on the CPS range from 0 to 6, with higher scores indicating more severe impairment. Values are shown in 2-year bins (i.e., the data point for 81 years of age contains data for ages 80 and 81), except for the last bin, which represents ages of 98, 99, and 100 years. No CPS data were available for carriers in the last age bin. Each data point represents the average CPS score for participants in the respective age bin. The I bars represent standard errors. The graph is based on 307 measurements from 53 carriers and 24,152 measurements from 3699 noncarriers. Patients in whom Alzheimer’s disease had been diagnosed were not included in the analysis.
Comment in
- Variant TREM2 as risk factor for Alzheimer's disease.
Neumann H, Daly MJ. Neumann H, et al. N Engl J Med. 2013 Jan 10;368(2):182-4. doi: 10.1056/NEJMe1213157. Epub 2012 Nov 14. N Engl J Med. 2013. PMID: 23151315 No abstract available. - Alzheimer disease: TREM2 linked to late-onset AD.
Jones B. Jones B. Nat Rev Neurol. 2013 Jan;9(1):5. doi: 10.1038/nrneurol.2012.254. Epub 2012 Dec 4. Nat Rev Neurol. 2013. PMID: 23208114 No abstract available. - TREM2: a new risk factor for Alzheimer's disease.
Singaraja RR. Singaraja RR. Clin Genet. 2013 Jun;83(6):525-6. doi: 10.1111/cge.12108. Clin Genet. 2013. PMID: 23347262 No abstract available. - TREM2 and neurodegenerative disease.
Jonsson T, Stefansson K. Jonsson T, et al. N Engl J Med. 2013 Oct 17;369(16):1568-9. doi: 10.1056/NEJMc1306509. N Engl J Med. 2013. PMID: 24131183 No abstract available. - TREM2 and neurodegenerative disease.
Reitz C, Mayeux R; Alzheimer’s Disease Genetics Consortium. Reitz C, et al. N Engl J Med. 2013 Oct 17;369(16):1564-5. doi: 10.1056/NEJMc1306509. N Engl J Med. 2013. PMID: 24131184 Free PMC article. No abstract available. - TREM2 and neurodegenerative disease.
Bertram L, Parrado AR, Tanzi RE. Bertram L, et al. N Engl J Med. 2013 Oct 17;369(16):1565. doi: 10.1056/NEJMc1306509. N Engl J Med. 2013. PMID: 24131185 No abstract available. - TREM2 and neurodegenerative disease.
Bird TD. Bird TD. N Engl J Med. 2013 Oct 17;369(16):1568. doi: 10.1056/NEJMc1306509. N Engl J Med. 2013. PMID: 24131188 No abstract available.
Similar articles
- TREM2 variants in Alzheimer's disease.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J; Alzheimer Genetic Analysis Group. Guerreiro R, et al. N Engl J Med. 2013 Jan 10;368(2):117-27. doi: 10.1056/NEJMoa1211851. Epub 2012 Nov 14. N Engl J Med. 2013. PMID: 23150934 Free PMC article. - R47H Variant of TREM2 Associated With Alzheimer Disease in a Large Late-Onset Family: Clinical, Genetic, and Neuropathological Study.
Korvatska O, Leverenz JB, Jayadev S, McMillan P, Kurtz I, Guo X, Rumbaugh M, Matsushita M, Girirajan S, Dorschner MO, Kiianitsa K, Yu CE, Brkanac Z, Garden GA, Raskind WH, Bird TD. Korvatska O, et al. JAMA Neurol. 2015 Aug;72(8):920-7. doi: 10.1001/jamaneurol.2015.0979. JAMA Neurol. 2015. PMID: 26076170 Free PMC article. - Coding variants in TREM2 increase risk for Alzheimer's disease.
Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, Norton JB, Hsu S, Harari O, Cai Y, Bertelsen S, Goate AM, Cruchaga C. Jin SC, et al. Hum Mol Genet. 2014 Nov 1;23(21):5838-46. doi: 10.1093/hmg/ddu277. Epub 2014 Jun 4. Hum Mol Genet. 2014. PMID: 24899047 Free PMC article. - TREM2 and the neuroimmunology of Alzheimer's disease.
Hickman SE, El Khoury J. Hickman SE, et al. Biochem Pharmacol. 2014 Apr 15;88(4):495-8. doi: 10.1016/j.bcp.2013.11.021. Epub 2013 Dec 16. Biochem Pharmacol. 2014. PMID: 24355566 Free PMC article. Review. - The role of TREM2 in Alzheimer's disease and other neurodegenerative disorders.
Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. Carmona S, et al. Lancet Neurol. 2018 Aug;17(8):721-730. doi: 10.1016/S1474-4422(18)30232-1. Epub 2018 Jul 17. Lancet Neurol. 2018. PMID: 30033062 Review.
Cited by
- Correlation between Blood Monocytes and CSF Tau in Alzheimer's Disease: The Effect of Gender and Cognitive Decline.
Cimiotti CGV, Paganetti P, Rossi S, Soldini E, Sacco L. Cimiotti CGV, et al. NeuroSci. 2023 Dec 12;4(4):319-330. doi: 10.3390/neurosci4040026. eCollection 2023 Dec. NeuroSci. 2023. PMID: 39484180 Free PMC article. - Peripheral innate immunophenotype in neurodegenerative disease: blood-based profiles and links to survival.
Strauss A, Swann P, Kigar SL, Christou R, Savinykh Yarkoni N, Turner L, Murley AG, Chouliaras L, Shapiro N, Ashton NJ, Savulich G, Bevan-Jones WR, Surendranthan A, Blennow K, Zetterberg H, O'Brien JT, Rowe JB, Malpetti M. Strauss A, et al. Mol Psychiatry. 2024 Oct 29. doi: 10.1038/s41380-024-02809-w. Online ahead of print. Mol Psychiatry. 2024. PMID: 39472664 - Cannabis Use and Cannabidiol Modulate HIV-Induced Alterations in TREM2 Expression: Implications for Age-Related Neuropathogenesis.
Avalos B, Kulbe JR, Ford MK, Laird AE, Walter K, Mante M, Florio JB, Boustani A, Chaillon A, Schlachetzki JCM, Sundermann EE, Volsky DJ, Rissman RA, Ellis RJ, Letendre SL, Iudicello J, Fields JA. Avalos B, et al. Viruses. 2024 Sep 24;16(10):1509. doi: 10.3390/v16101509. Viruses. 2024. PMID: 39459844 Free PMC article. - The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.
Fanlo-Ucar H, Picón-Pagès P, Herrera-Fernández V, Ill-Raga G, Muñoz FJ. Fanlo-Ucar H, et al. Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review. - Preclinical and first-in-human evaluation of AL002, a novel TREM2 agonistic antibody for Alzheimer's disease.
Long H, Simmons A, Mayorga A, Burgess B, Nguyen T, Budda B, Rychkova A, Rhinn H, Tassi I, Ward M, Yeh F, Schwabe T, Paul R, Kenkare-Mitra S, Rosenthal A. Long H, et al. Alzheimers Res Ther. 2024 Oct 23;16(1):235. doi: 10.1186/s13195-024-01599-1. Alzheimers Res Ther. 2024. PMID: 39444037 Free PMC article. Clinical Trial.
References
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. - PubMed
- Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01HG004438/HG/NHGRI NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01HG004610/HG/NHGRI NIH HHS/United States
- U01 HG004603/HG/NHGRI NIH HHS/United States
- U01AG006781/AG/NIA NIH HHS/United States
- P50-AG025688/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- U01HG004603/HG/NHGRI NIH HHS/United States
- U01 HG004610/HG/NHGRI NIH HHS/United States
- U01 HG006375/HG/NHGRI NIH HHS/United States
- U01 HG004438/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical