Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes - PubMed (original) (raw)
Live virus-free or die: coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes
Kira S Makarova et al. Biol Direct. 2012.
Abstract
Background: The virus-host arms race is a major theater for evolutionary innovation. Archaea and bacteria have evolved diverse, elaborate antivirus defense systems that function on two general principles: i) immune systems that discriminate self DNA from nonself DNA and specifically destroy the foreign, in particular viral, genomes, whereas the host genome is protected, or ii) programmed cell suicide or dormancy induced by infection.
Presentation of the hypothesis: Almost all genomic loci encoding immunity systems such as CRISPR-Cas, restriction-modification and DNA phosphorothioation also encompass suicide genes, in particular those encoding known and predicted toxin nucleases, which do not appear to be directly involved in immunity. In contrast, the immunity systems do not appear to encode antitoxins found in typical toxin-antitoxin systems. This raises the possibility that components of the immunity system themselves act as reversible inhibitors of the associated toxin proteins or domains as has been demonstrated for the Escherichia coli anticodon nuclease PrrC that interacts with the PrrI restriction-modification system. We hypothesize that coupling of diverse immunity and suicide/dormancy systems in prokaryotes evolved under selective pressure to provide robustness to the antivirus response. We further propose that the involvement of suicide/dormancy systems in the coupled antivirus response could take two distinct forms:1) induction of a dormancy-like state in the infected cell to 'buy time' for activation of adaptive immunity; 2) suicide or dormancy as the final recourse to prevent viral spread triggered by the failure of immunity.
Testing the hypothesis: This hypothesis entails many experimentally testable predictions. Specifically, we predict that Cas2 protein present in all cas operons is a mRNA-cleaving nuclease (interferase) that might be activated at an early stage of virus infection to enable incorporation of virus-specific spacers into the CRISPR locus or to trigger cell suicide when the immune function of CRISPR-Cas systems fails. Similarly, toxin-like activity is predicted for components of numerous other defense loci.
Implications of the hypothesis: The hypothesis implies that antivirus response in prokaryotes involves key decision-making steps at which the cell chooses the path to follow by sensing the course of virus infection.
Reviewers: This article was reviewed by Arcady Mushegian, Etienne Joly and Nick Grishin. For complete reviews, go to the Reviewers' reports section.
Figures
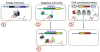
Figure 1
The defense systems in prokaryotes: innate immunity, adaptive immunity and programmed suicide/dormancy.
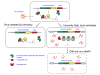
Figure 2
Organization of the genomic loci that encode prokaryotic immune systems including toxin genes. The core genes of CRISPR-Cas, RM, and DND systems in predicted operons are shown by pink arrows; genes with (predicted) toxin activity are shown by different colors, and the (predicted) toxin domains are denoted with red outline. The Csa3 protein in the Type IA system lacks the HEPN domain. Abbreviations: HEPN - higher eukarytoes and prokaryotes nucleotide-binding domain; Sir2, ParB and REase, DEDD are nucleases from distinct superfamilies. A. CRISPR-Cas. Gene names follow the nomenclature and classification from [18]. B. Restriction-modification. Gene names follow the nomenclature and classification from [46]. C. Phosphorothioation. Gene names follow the nomenclature from [12]. D. Prokaryotic argonaut, pAgo.
Figure 3
The immunity-dormancy/suicide coupling hypothesis: Route 1 – toxin action before immunity activation. The coupling between dormancy-suicide and immunity is specifically illustrated by the CRISPR-Cas system that is hypothesized to adapt by inserting a cognate spacer during a dormancy-like phase induced by the action of the toxin (typically, Cas2).
Figure 4
The immunity-dormancy/suicide coupling hypothesis: Route 2 – toxin action after immunity failure. The coupling between dormancy-suicide and immunity is illustrated by the CRISPR-Cas system as in Figure 3.
Similar articles
- CRISPR-Cas: evolution of an RNA-based adaptive immunity system in prokaryotes.
Koonin EV, Makarova KS. Koonin EV, et al. RNA Biol. 2013 May;10(5):679-86. doi: 10.4161/rna.24022. Epub 2013 Feb 25. RNA Biol. 2013. PMID: 23439366 Free PMC article. Review. - Evolutionary Genomics of Defense Systems in Archaea and Bacteria.
Koonin EV, Makarova KS, Wolf YI. Koonin EV, et al. Annu Rev Microbiol. 2017 Sep 8;71:233-261. doi: 10.1146/annurev-micro-090816-093830. Epub 2017 Jun 28. Annu Rev Microbiol. 2017. PMID: 28657885 Free PMC article. Review. - Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems.
Makarova KS, Aravind L, Wolf YI, Koonin EV. Makarova KS, et al. Biol Direct. 2011 Jul 14;6:38. doi: 10.1186/1745-6150-6-38. Biol Direct. 2011. PMID: 21756346 Free PMC article. - Coupling immunity and programmed cell suicide in prokaryotes: Life-or-death choices.
Koonin EV, Zhang F. Koonin EV, et al. Bioessays. 2017 Jan;39(1):1-9. doi: 10.1002/bies.201600186. Epub 2016 Nov 29. Bioessays. 2017. PMID: 27896818 Review. - Viral diversity threshold for adaptive immunity in prokaryotes.
Weinberger AD, Wolf YI, Lobkovsky AE, Gilmore MS, Koonin EV. Weinberger AD, et al. mBio. 2012 Dec 4;3(6):e00456-12. doi: 10.1128/mBio.00456-12. mBio. 2012. PMID: 23221803 Free PMC article.
Cited by
- Protospacer recognition motifs: mixed identities and functional diversity.
Shah SA, Erdmann S, Mojica FJ, Garrett RA. Shah SA, et al. RNA Biol. 2013 May;10(5):891-9. doi: 10.4161/rna.23764. Epub 2013 Feb 12. RNA Biol. 2013. PMID: 23403393 Free PMC article. - What bacterial cell death teaches us about life.
Johnson AG, Kranzusch PJ. Johnson AG, et al. PLoS Pathog. 2022 Oct 27;18(10):e1010879. doi: 10.1371/journal.ppat.1010879. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36301823 Free PMC article. No abstract available. - Restriction endonuclease cleavage of phage DNA enables resuscitation from Cas13-induced bacterial dormancy.
Williams MC, Reker AE, Margolis SR, Liao J, Wiedmann M, Rojas ER, Meeske AJ. Williams MC, et al. Nat Microbiol. 2023 Mar;8(3):400-409. doi: 10.1038/s41564-022-01318-2. Epub 2023 Feb 13. Nat Microbiol. 2023. PMID: 36782027 Free PMC article. - RNA-Targeting CRISPR-Cas Systems and Their Applications.
Burmistrz M, Krakowski K, Krawczyk-Balska A. Burmistrz M, et al. Int J Mol Sci. 2020 Feb 7;21(3):1122. doi: 10.3390/ijms21031122. Int J Mol Sci. 2020. PMID: 32046217 Free PMC article. Review. - Comprehensive search for accessory proteins encoded with archaeal and bacterial type III CRISPR-cas gene cassettes reveals 39 new cas gene families.
Shah SA, Alkhnbashi OS, Behler J, Han W, She Q, Hess WR, Garrett RA, Backofen R. Shah SA, et al. RNA Biol. 2019 Apr;16(4):530-542. doi: 10.1080/15476286.2018.1483685. Epub 2018 Jun 19. RNA Biol. 2019. PMID: 29911924 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials