Eosinophils: changing perspectives in health and disease - PubMed (original) (raw)
Review
Eosinophils: changing perspectives in health and disease
Helene F Rosenberg et al. Nat Rev Immunol. 2013 Jan.
Abstract
Eosinophils have been traditionally perceived as terminally differentiated cytotoxic effector cells. Recent studies have profoundly altered this simplistic view of eosinophils and their function. New insights into the molecular pathways that control the development, trafficking and degranulation of eosinophils have improved our understanding of the immunomodulatory functions of these cells and their roles in promoting homeostasis. Likewise, recent developments have generated a more sophisticated view of how eosinophils contribute to the pathogenesis of different diseases, including asthma and primary hypereosinophilic syndromes, and have also provided us with a more complete appreciation of the activities of these cells during parasitic infection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
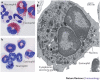
Figure 1. The eosinophil.
a | Human eosinophils from peripheral blood stained with modified Giemsa exhibit characteristic bilobed nuclei and large red-stained cytoplasmic secretory granules. The cells with multilobed nuclei and without large granules are neutrophils. Original magnification, ×100. b | The image shows eosinophils and neutrophils isolated from the spleen of a _Cd2_-interleukin-5-transgenic mouse and stained with modified Giemsa. c | The image shows a transmission electron micrograph of a mouse eosinophil. Cytoplasmic secretory granules are indicated by the arrows; the central core of these granules contains cationic major basic protein, and their periphery contains the remaining major cationic proteins, cytokines, chemokines, growth factors and enzymes. Original magnification, ×6,000.
Figure 2. Cellular features of eosinophils.
Eosinophils are equipped with features that promote interactions with the environment. In one such interaction, eosinophils release the contents of their specific granules in response to external stimuli. Some of these granule contents are released via membrane-bound vesicles known as eosinophil sombrero vesicles. Eosinophils also synthesize lipid mediators for release in cytoplasmic lipid bodies and store Charcot–Leyden crystal protein (CLC) in primary granules. Although not highly biosynthetic, mature eosinophils have minimal numbers of mitochondria and a limited endoplasmic reticulum (ER) and Golgi, as well as a nucleus. Eosinophils express a wide variety of receptors that modulate adhesion, growth, survival, activation, migration and pattern recognition. Mouse eosinophils do not express CLC or Fcε receptor 1 (FcεR1) and have divergent homologues of sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8) and the granule ribonucleases eosinophil-derived neurotoxin (EDN) and eosinophil cationic protein (ECP). APRIL, a proliferation-inducing ligand; CCL, CC-chemokine ligand; CCR, CC-chemokine receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; EGF, epidermal growth factor; EPX, eosinophil peroxidase; FPR1, formyl peptide receptor 1; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MBP, major basic protein; NGF, nerve growth factor; NOD, nucleotide-binding oligomerization domain protein; PAR, proteinase-activated receptor; PDGF, platelet-derived growth factor; PIRB, paired immunoglobulin-like receptor B; PPARγ, peroxisome proliferator-activated receptor-γ; PRR, pattern-recognition receptor; PSGL1, P-selectin glycoprotein ligand 1; RAGE, receptor for advanced glycation end-products; RIG-I, retinoic acid-inducible gene I; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumour necrosis factor; SCF, stem cell factor; VEGF, vascular endothelial growth factor.
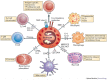
Figure 3. Eosinophils modulate the function of other leukocytes.
Eosinophils not only respond to signals, but also have a definitive impact on the actions of other leukocytes. Eosinophils can express MHC class II and co-stimulatory molecules, process antigens and stimulate T cells to proliferate and produce cytokines in an antigen-specific manner. Furthermore, acting together with dendritic cells (DCs), eosinophils regulate the recruitment of T helper 2 (TH2) cells in response to allergen sensitization and challenge by producing CC-chemokine ligand 17 (CCL17) and CCL22 (Refs 40, 41). Eosinophils also prime B cells for antigen-specific IgM production and sustain long-lived plasma cells in mouse bone marrow via the production of a proliferation-inducing ligand (APRIL) and interleukin-6 (IL-6),. Eosinophils that are stimulated by CpG DNA induce DC maturation51. Indeed, the eosinophil granule protein eosinophil-derived neurotoxin (EDN) promotes the maturation and activation of DCs,. Major basic protein (MBP) released from eosinophils activates neutrophils, causing them to release superoxide and IL-8 and increase their expression of the cell-surface integrin complement receptor 3 (CR3). Eosinophils also maintain alternatively activated macrophages in adipose tissue by producing IL-4 and IL-13 (Ref. 50). The eosinophil granule proteins MBP, eosinophil cationic protein (ECP) and eosinophil peroxidase (EPX) activate mast cells, resulting in the release of histamine. Likewise, eosinophil-derived nerve growth factor (NGF) prolongs mast cell survival.
Comment in
- Primary lysis of eosinophils as a major mode of activation of eosinophils in human diseased tissues.
Persson C, Uller L. Persson C, et al. Nat Rev Immunol. 2013 Dec;13(12):902. doi: 10.1038/nri3341-c1. Nat Rev Immunol. 2013. PMID: 24270781 No abstract available. - Reply to eosinophil cytolysis and release of cell-free granules.
Rosenberg HF, Foster PS. Rosenberg HF, et al. Nat Rev Immunol. 2013 Dec;13(12):902. doi: 10.1038/nri3341-c2. Nat Rev Immunol. 2013. PMID: 24270783 No abstract available.
Similar articles
- The eosinophil, a cell with multiple facets.
Capron M, Goldman M. Capron M, et al. Therapie. 2001 Jul-Aug;56(4):371-5. Therapie. 2001. PMID: 11677855 Review. No abstract available. - Changing roles of eosinophils in health and disease.
Furuta GT, Atkins FD, Lee NA, Lee JJ. Furuta GT, et al. Ann Allergy Asthma Immunol. 2014 Jul;113(1):3-8. doi: 10.1016/j.anai.2014.04.002. Epub 2014 May 1. Ann Allergy Asthma Immunol. 2014. PMID: 24795292 Free PMC article. Review. - The Eosinophil in Health and Disease: from Bench to Bedside and Back.
Liao W, Long H, Chang CC, Lu Q. Liao W, et al. Clin Rev Allergy Immunol. 2016 Apr;50(2):125-39. doi: 10.1007/s12016-015-8507-6. Clin Rev Allergy Immunol. 2016. PMID: 26410377 Review. - Eosinophils, allergy and parasites.
Dombrowicz D, Capron M. Dombrowicz D, et al. Curr Opin Immunol. 2001 Dec;13(6):716-20. doi: 10.1016/s0952-7915(01)00284-9. Curr Opin Immunol. 2001. PMID: 11677095 Review. - Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders.
Ackerman SJ, Bochner BS. Ackerman SJ, et al. Immunol Allergy Clin North Am. 2007 Aug;27(3):357-75. doi: 10.1016/j.iac.2007.07.004. Immunol Allergy Clin North Am. 2007. PMID: 17868854 Free PMC article. Review.
Cited by
- Image-Level Uncertainty in Pseudo-Label Selection for Semi-Supervised Segmentation.
McBee P, Zulqarnain F, Syed S, Brown DE. McBee P, et al. Annu Int Conf IEEE Eng Med Biol Soc. 2022 Jul;2022:4740-4744. doi: 10.1109/EMBC48229.2022.9871359. Annu Int Conf IEEE Eng Med Biol Soc. 2022. PMID: 36086227 Free PMC article. - Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation.
Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, Schmid I, Radonjic-Hoesli S, Boutet S, Williams GJ, Messerschmidt M, Seibert MM, Cascio D, Zatsepin NA, Burghammer M, Riekel C, Colletier JP, Riek R, Eisenberg DS, Simon HU. Soragni A, et al. Mol Cell. 2015 Mar 19;57(6):1011-1021. doi: 10.1016/j.molcel.2015.01.026. Epub 2015 Feb 26. Mol Cell. 2015. PMID: 25728769 Free PMC article. - Eosinophilic inflammation promotes CCL6-dependent metastatic tumor growth.
Li F, Du X, Lan F, Li N, Zhang C, Zhu C, Wang X, He Y, Shao Z, Chen H, Luo M, Li W, Chen Z, Ying S, Shen H. Li F, et al. Sci Adv. 2021 May 26;7(22):eabb5943. doi: 10.1126/sciadv.abb5943. Print 2021 May. Sci Adv. 2021. PMID: 34039594 Free PMC article. - Divergent Siglec-F(eights) of mouse and human eosinophil death.
Jacobsen EA. Jacobsen EA. J Leukoc Biol. 2020 Jul;108(1):9-11. doi: 10.1002/JLB.5CE0520-108R. Epub 2020 Jun 18. J Leukoc Biol. 2020. PMID: 32557797 Free PMC article. - Eosinophils, probiotics, and the microbiome.
Rosenberg HF, Masterson JC, Furuta GT. Rosenberg HF, et al. J Leukoc Biol. 2016 Nov;100(5):881-888. doi: 10.1189/jlb.3RI0416-202R. Epub 2016 Aug 22. J Leukoc Biol. 2016. PMID: 27549754 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- ZIA AI000941/ImNIH/Intramural NIH HHS/United States
- Z01 AI000943/ImNIH/Intramural NIH HHS/United States
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- ZIA AI000942/ImNIH/Intramural NIH HHS/United States
- AI000941/AI/NIAID NIH HHS/United States
- ZIA AI000943/ImNIH/Intramural NIH HHS/United States
- Z01 AI000941/ImNIH/Intramural NIH HHS/United States
- AI000943/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources