Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature - PubMed (original) (raw)
. 2013 Jan 24;121(4):679-91.
doi: 10.1182/blood-2012-04-426734. Epub 2012 Nov 15.
Peter Kraft, Angela Dreykluft, Ina Hagedorn, Kerstin Göbel, Michael K Schuhmann, Friederike Langhauser, Xavier Helluy, Tobias Schwarz, Stefan Bittner, Christian T Mayer, Marc Brede, Csanad Varallyay, Mirko Pham, Martin Bendszus, Peter Jakob, Tim Magnus, Sven G Meuth, Yoichiro Iwakura, Alma Zernecke, Tim Sparwasser, Bernhard Nieswandt, Guido Stoll, Heinz Wiendl
Affiliations
- PMID: 23160472
- PMCID: PMC3790947
- DOI: 10.1182/blood-2012-04-426734
Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature
Christoph Kleinschnitz et al. Blood. 2013.
Abstract
We have recently identified T cells as important mediators of ischemic brain damage, but the contribution of the different T-cell subsets is unclear. Forkhead box P3 (FoxP3)-positive regulatory T cells (Tregs) are generally regarded as prototypic anti-inflammatory cells that maintain immune tolerance and counteract tissue damage in a variety of immune-mediated disorders. In the present study, we examined the role of Tregs after experimental brain ischemia/reperfusion injury. Selective depletion of Tregs in the DEREG mouse model dramatically reduced infarct size and improved neurologic function 24 hours after stroke and this protective effect was preserved at later stages of infarct development. The specificity of this detrimental Treg effect was confirmed by adoptive transfer experiments in wild-type mice and in Rag1(-/-) mice lacking lymphocytes. Mechanistically, Tregs induced microvascular dysfunction in vivo by increased interaction with the ischemic brain endothelium via the LFA-1/ICAM-1 pathway and platelets and these findings were confirmed in vitro. Ablation of Tregs reduced microvascular thrombus formation and improved cerebral reperfusion on stroke, as revealed by ultra-high-field magnetic resonance imaging at 17.6 Tesla. In contrast, established immunoregulatory characteristics of Tregs had no functional relevance. We define herein a novel and unexpected role of Tregs in a primary nonimmunologic disease state.
Figures
Figure 1
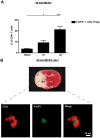
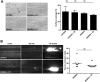
Tregs are present in the ischemic brain early after stroke and are mainly found in the vascular compartment. (A) Flow cytometric analysis of FoxP3+ Tregs counted in the ischemic hemispheres on day 1 (d1) and d3 after 60 minutes of tMCAO or sham-operated mice. Transcardial perfusion of animals was omitted before brain sampling. (B) Top panel is a macroscopic view of a representative TTC-stained brain section from a regular DEREG mouse without diphtheria toxin treatment on day 1 after 60 minutes of tMCAO showing that the immunohistochemical pictures shown at the bottom of the figure were taken from the basal ganglia (BG). Bottom panel is immunohistochemical brain sections from DEREG mice on day 1 after 60 minutes of tMCAO showing FoxP3+ Tregs predominantly in the cerebral vasculature (double staining with the endothelial marker CD31). The area of the basal ganglia is depicted. C indicates cortex. *P < .05; ***P < .0001.
Figure 2
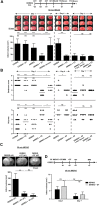
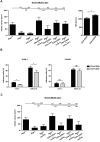
Treg depletion protects from acute ischemic stroke. (A) Infarct volumes on day 1 or day 4 after 60 minutes of tMCAO in Treg-depleted DEREG mice (DEREG + DT) and controls or Treg-depleted DEREG mice treated with anti–neutrophil serum (ANS) or control serum as calculated from TTC-stained brain sections. DT indicates diphtheria toxin (B) Neurologic deficits after stroke (day 1 or day 4) were assessed by the Bederson score and the grip test score. Consistent with infarct volume reduction, Treg-depleted DEREG mice also had a significantly better functional outcome. (C) Serial coronal MR brain images (1.5 Tesla) confirmed smaller infarct volumes in Treg-depleted DEREG mice on day 1 after 60 minutes of tMCAO and excluded secondary infarct growth in these mice until day 7. (D) Delayed depletion of Tregs in DEREG mice (DEREG + DT) also did not lead to larger infarcts on day 7 (30 minutes of tMCAO). Alleged shrinkage of strokes between day 1 and day 7 is because of fogging effects occurring during infarct maturation. *P < .05, **P < .001, and ***P < .0001 between the indicated groups; ns indicates not significant.
Figure 3
Tregs exacerbate ischemic brain damage in wild-type mice and _Rag1_−/− mice lacking T cells independently of Treg immunologic function. (A) Purified CD4+CD25+ Tregs (750 000) were transferred into regular C57Bl/6 mice 24 hours before 30 minutes of tMCAO and infarct volumes were determined on day 1. (B) The stroke-protective effect observed in _Rag1_−/− mice on day 1 after 60 minutes of tMCAO could be reversed by adoptive transfer of Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) or non-Tregs (CD4+CD25− or CD4+CD25−FoxP3−). (C) Adoptive transfer of “wannabe” Tregs (CD4+CD25+ or CD4+CD25+FoxP3+) from DEREG × scurfy mice into _Rag1_−/− mice also induced infarctions of regular size on day 1 after 60 minutes of tMCAO, indicating that the immunologic function of Tregs is dispensable for stroke development. (D) CD4+CD25+ Tregs (750 000) were collected from _Il-17_−/− mice, _Ifn_γ−/− mice, and _Ccr6_−/− mice and transferred into _Rag1_−/− mice 24 hours before 60 minutes of tMCAO. Infarct volumes were determined on day 1. The detrimental effects of Tregs in ischemic stroke cannot be ascribed to one specific cytokine because infarct volumes after adoptive transfer were similar to those observed in Rag1+/+ mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
Figure 4
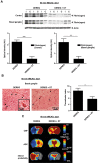
Depletion of Tregs reduces intracerebral thrombosis and improves cerebral blood flow after stroke. (A) Accumulation of fibrin(ogen) in the infarcted (I) and contralateral (C) cortices and basal ganglia of naive DEREG mice and Treg-depleted DEREG mice (DEREG + DT) was analyzed by immunoblotting 23 hours after 60 minutes of tMCAO and quantified. Three representative immunoblots of each group are shown. The higher fibrin(ogen) signal detectable also in the healthy contralateral (= left) hemisphere of nondepleted DEREG mice is probably a consequence of increased thrombotic activity in this region because of massive ipsilateral infarct swelling. DT indicates diphtheria toxin; AU, arbitrary units. (B) The number of thrombotic vessels (thrombosis index) in the ischemic basal ganglia of DEREG mice and Treg-depleted DEREG mice was counted from H&E-stained brain sections on day 1 after 60 minutes of tMCAO. Although numerous occlusions of vessel lumina were found in naive DEREG mice (arrows), the microvascular patency was significantly increased in the absence of Tregs (arrowheads). (C) Reduced intracerebral thrombosis was related to improved CBF, fewer tissue infarctions as reflected by lower apparent diffusion coefficient (ADC) values, and a lower infarct probability in Treg-depleted DEREG mice as revealed by MRI at ultra-high-field strength (17.6 Tesla) on day 1 after 60 minutes of tMCAO. *P < .05, **P < .001, and ***P < .0001 between the indicated groups.
Figure 5
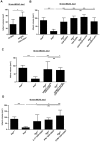
Depletion of Tregs does not induce a general defect in platelet function or activation. (A) Platelets in whole blood from Treg-depleted DEREG mice (DEREG + DT) form stable thrombi when perfused over a collagen-coated surface at a shear rate of 1000/s. On the left are representative phase-contrast images. On the right is the mean surface coverage by thrombi in the indicated mouse groups. DT indicates diphtheria toxin; and ns, not significant. (B) In vivo analysis of thrombus formation in Treg-depleted DEREG mice (DEREG + DT) and controls. Mesenteric arterioles were treated with FeCl3 and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo fluorescence microscopy. Representative images (left) and the time to vessel occlusion (right) are shown. Each symbol represents one individual. ns indicates not significant. Data are presented as means ± SD or scatter plots showing the median (panel B).
Figure 6
Tregs interact with cerebral endothelial cells and platelets to promote ischemic neurodegeneration after stroke. (A left) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into _Rag1_−/− mice 24 hours before 60 minutes of tMCAO in the presence of anti–LFA-1 blocking Abs or control Abs. Infarct volumes were determined on day 1. Blocking of LFA-1 reversed the stroke-enhancing effect of CD4+CD25+ Tregs but not of CD4+CD25− non-Tregs in _Rag1_−/− mice. Right, circulating CD4+CD25+ Tregs express higher amounts of LFA-1 than CD4+CD25− non-Tregs on day 1 after 60 minutes of tMCAO. MFI indicates mean fluorescence intensity. (B) In vitro binding capacity to ICAM-1 (left) but not VCAM-1 (right) is more pronounced in CD4+CD25+ Tregs than in CD4+CD25− non-Tregs both under basal conditions and after stimulation with CXCL12. (C) Purified CD4+CD25+ (750 000) Tregs or CD4+CD25− non-Tregs were transferred into _Rag1_−/− mice 24 hours before 60 minutes of tMCAO. _Rag1_−/− mice were treated with antiplatelet serum to deplete circulating platelets or controls serum and infarct volumes were determined on day 1. Depletion of platelets reversed the stroke-enhancing effect of CD4+CD25+ Tregs and also of CD4+CD25− non-Tregs in _Rag1_−/− mice. *P < .05 and ***P < .0001 between the indicated groups; ns indicates not significant.
Similar articles
- Activated regulatory T-cells attenuate myocardial ischaemia/reperfusion injury through a CD39-dependent mechanism.
Xia N, Jiao J, Tang TT, Lv BJ, Lu YZ, Wang KJ, Zhu ZF, Mao XB, Nie SF, Wang Q, Tu X, Xiao H, Liao YH, Shi GP, Cheng X. Xia N, et al. Clin Sci (Lond). 2015 May 1;128(10):679-93. doi: 10.1042/CS20140672. Clin Sci (Lond). 2015. PMID: 25558978 - Adoptive regulatory T-cell therapy protects against cerebral ischemia.
Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, Liang W, Thomson AW, Chen J, Hu X. Li P, et al. Ann Neurol. 2013 Sep;74(3):458-71. doi: 10.1002/ana.23815. Epub 2013 May 14. Ann Neurol. 2013. PMID: 23674483 Free PMC article. - In Vivo Expansion of Regulatory T Cells with IL-2/IL-2 Antibody Complex Protects against Transient Ischemic Stroke.
Zhang H, Xia Y, Ye Q, Yu F, Zhu W, Li P, Wei Z, Yang Y, Shi Y, Thomson AW, Chen J, Hu X. Zhang H, et al. J Neurosci. 2018 Nov 21;38(47):10168-10179. doi: 10.1523/JNEUROSCI.3411-17.2018. Epub 2018 Oct 5. J Neurosci. 2018. PMID: 30291203 Free PMC article. - Diverse functions and mechanisms of regulatory T cell in ischemic stroke.
Wu Y, Li J, Shou J, Zhang W, Chen C. Wu Y, et al. Exp Neurol. 2021 Sep;343:113782. doi: 10.1016/j.expneurol.2021.113782. Epub 2021 Jun 8. Exp Neurol. 2021. PMID: 34116055 Review. - Regulatory T Cells: Pathophysiological Roles and Clinical Applications.
Sakai R, Komai K, Iizuka-Koga M, Yoshimura A, Ito M. Sakai R, et al. Keio J Med. 2020 Mar 25;69(1):1-15. doi: 10.2302/kjm.2019-0003-OA. Epub 2019 Jul 26. Keio J Med. 2020. PMID: 31353330 Review.
Cited by
- Inhibition of PCSK9 Protects against Cerebral Ischemia‒Reperfusion Injury via Attenuating Microcirculatory Dysfunction.
Luo Y, Yuan L, Liu Z, Dong W, Huang L, Liao A, Xie Y, Liu R, Lan W, Cai Y, Zhu W. Luo Y, et al. Neurochem Res. 2024 Nov 16;50(1):10. doi: 10.1007/s11064-024-04272-z. Neurochem Res. 2024. PMID: 39548030 - Peripheral Lymphocyte-to-Monocyte Ratio as a Predictive Factor for Early Neurological Deterioration in Patients with Acute Ischemic Stroke.
Sun L, Ye X, Yu J, Wang L, Wu Y, Cui J, Dai L. Sun L, et al. Int J Gen Med. 2024 Sep 27;17:4397-4405. doi: 10.2147/IJGM.S483064. eCollection 2024. Int J Gen Med. 2024. PMID: 39355340 Free PMC article. - The Role of Inflammation in the Pathogenesis of Cardiogenic Shock Secondary to Acute Myocardial Infarction: A Narrative Review.
Kologrivova I, Kercheva M, Panteleev O, Ryabov V. Kologrivova I, et al. Biomedicines. 2024 Sep 11;12(9):2073. doi: 10.3390/biomedicines12092073. Biomedicines. 2024. PMID: 39335587 Free PMC article. Review. - Systemic immune-inflammatory markers and long-term prognosis after revascularization in Moyamoya disease: a retrospective study.
Wang S, Liu W, Zhai Y, Liu C, Ge P, Zhang D. Wang S, et al. Front Neurol. 2024 Sep 2;15:1418729. doi: 10.3389/fneur.2024.1418729. eCollection 2024. Front Neurol. 2024. PMID: 39286803 Free PMC article. - The Role of Thrombo-inflammation in Ischemic Stroke: Focus on the Manipulation and Clinical Application.
Luo Y, Dong W, Yuan L, Zhu YA, Zhang DD, Ni H, Zhu W. Luo Y, et al. Mol Neurobiol. 2024 Aug 6. doi: 10.1007/s12035-024-04397-w. Online ahead of print. Mol Neurobiol. 2024. PMID: 39107669 Review.
References
- Magnus T, Wiendl H, Kleinschnitz C. Immune mechanisms of stroke. Curr Opin Neurol. 2012;25(3):334–340. - PubMed
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56(2):149–171. - PubMed
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15(1):42–51. - PubMed
- Liesz A, Zhou W, Mracskó É, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134(Pt 3):704–720. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases