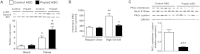Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats - PubMed (original) (raw)
. 2013 May;57(5):1763-72.
doi: 10.1002/hep.26170. Epub 2013 Jan 25.
Toru Yoshimura, Jennifer L Cantley, Sachin K Majumdar, Fitsum Guebre-Egziabher, Romy Kursawe, Daniel F Vatner, Ioana Fat, Mario Kahn, Derek M Erion, Xian-Man Zhang, Dongyan Zhang, Vara Prasad Manchem, Sanjay Bhanot, Glenn S Gerhard, Kitt F Petersen, Gary W Cline, Varman T Samuel, Gerald I Shulman
Affiliations
- PMID: 23175050
- PMCID: PMC3597437
- DOI: 10.1002/hep.26170
Free PMC article
Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats
Naoki Kumashiro et al. Hepatology. 2013 May.
Free PMC article
Abstract
Genome-wide array studies have associated the patatin-like phospholipase domain-containing 3 (PNPLA3) gene polymorphisms with hepatic steatosis. However, it is unclear whether PNPLA3 functions as a lipase or a lipogenic enzyme and whether PNPLA3 is involved in the pathogenesis of hepatic insulin resistance. To address these questions we treated high-fat-fed rats with specific antisense oligonucleotides to decrease hepatic and adipose pnpla3 expression. Reducing pnpla3 expression prevented hepatic steatosis, which could be attributed to decreased fatty acid esterification measured by the incorporation of [U-(13) C]-palmitate into hepatic triglyceride. While the precursors for phosphatidic acid (PA) (long-chain fatty acyl-CoAs and lysophosphatidic acid [LPA]) were not decreased, we did observe an ∼20% reduction in the hepatic PA content, ∼35% reduction in the PA/LPA ratio, and ∼60%-70% reduction in transacylation activity at the level of acyl-CoA:1-acylglycerol-sn-3-phosphate acyltransferase. These changes were associated with an ∼50% reduction in hepatic diacylglycerol (DAG) content, an ∼80% reduction in hepatic protein kinase Cε activation, and increased hepatic insulin sensitivity, as reflected by a 2-fold greater suppression of endogenous glucose production during the hyperinsulinemic-euglycemic clamp. Finally, in humans, hepatic PNPLA3 messenger RNA (mRNA) expression was strongly correlated with hepatic triglyceride and DAG content, supporting a potential lipogenic role of PNPLA3 in humans.
Conclusion: PNPLA3 may function primarily in a lipogenic capacity and inhibition of PNPLA3 may be a novel therapeutic approach for treatment of nonalcoholic fatty liver disease-associated hepatic insulin resistance.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures
Fig 1
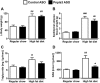
Pnpla3 ASO suppressed pnpla3 expression in liver and white adipose tissue. (A) Hepatic pnpla3 mRNA expression in overnight fasted (O/N fasted) or 5 hours refed condition in regular chow or HFF rats (n = 4-9 per group). The reduction percent in pnpla3 ASO rats compared with control ASO-treated rats in the same condition is shown. *P < 0.05, **_P_ < 0.01, ***_P_ < 0.001 compared with control ASO rats in the same condition, #_P_ < 0.05, ###_P_ < 0.001 compared between O/N fasted and refed condition in control ASO rats treated with the same diet. P < 0.01 compared between regular chow and high-fat diet condition in O/N fasted control ASO-treated rats. (B) Hepatic pnpla3 protein expressions in O/N fasted HFF rats (n = 5-6 per group). *P < 0.05 compared with control ASO rats. (C) Pnpla3 mRNA expression in epididymal adipose tissue in refed regular chow fed rats (n = 4 per group). ***P < 0.001 compared with control ASO rats. All data are expressed as mean ± SEM.
Fig 2
Pnpla3 ASO decreased hepatic lipid content in HFF rats. (A) Increase in body weight during the treatments in regular chow-fed and HFF rats treated with either a control or Pnpla3 ASO (n = 5-11 per group). (B-D) Epididymal adipose tissue weight, hepatic triglyceride content, hepatic DAG content, respectively, at sacrifice (n = 5-11 per group). #P < 0.05, ##P < 0.01, ###P < 0.001 compared to control ASO rats in regular chow fed condition. *P < 0.05 compared with control ASO rats in HFF condition. All data are expressed as mean ± SEM.
Fig 3
Pnpla3 ASO increased hepatic insulin sensitivity in HFF rats. (A) Basal endogenous glucose production (n = 9-10 per group). (B,C) Endogenous glucose production and percent suppression of endogenous glucose production during hyperinsulinemic-euglycemic clamps, respectively (n = 9-10 per group). ***P < 0.001 compared with control ASO-treated rats. All data are expressed as mean ± SEM.
Fig 4
Pnpla3 ASO improved hepatic insulin signaling accompanied with a decrease in hepatic membrane DAG content and PKCε activation. (A) Akt phosphorylation (Ser473) assay in HFF rats (n = 4 for basal control ASO rats and n = 6 for the other groups), #P < 0.05 and ##P < 0.01 compared with control ASO rats in basal condition. *P < 0.05 compared with control ASO rats in clamp condition. (B) Membrane DAG content (n = 5-6 per group), ##P < 0.01 compared with control ASO rats in regular chow fed condition. *P < 0.05 compared with control ASO rats in HFF condition. (C) PKCε translocation assay in HFF rats (n = 6 per group), ***P < 0.001 compared with control ASO rats. All data are expressed as mean ± SEM.
Fig 5
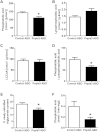
PNPLA3 ASO decreased hepatic fatty acid esterification in HFF rats. (A-C) Hepatic phosphatidic acid, lysophosphatidic acid, and long-chain fatty acyl-CoA (LCCoA) content, respectively (n = 6 per group). (D) Hepatic phosphatidic acid / lysophosphatidic acid ratio (n = 6 per group). (E) In vivo hepatic fatty acid esterification assay (n = 7 per group). (F) Lysophosphatidic acid acyltransferase activity assay (n = 6 per group). Protein samples were extracted from total liver lysate using flash-frozen livers, which are the same livers used for knockdown confirmation, lipid content, and PKCε assays in HFF overnight fasted condition, then incubated with 14C-palmitoyl CoA and lysophosphatidic acid. Produced 14C-labeled phosphatidic acid was measured with a scintillation counter. *P < 0.05 compared to control ASO rats. All data are expressed as mean ± SEM.
Fig 6
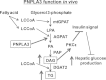
The lipogenic role of PNPLA3 on hepatic steatosis and hepatic insulin resistance in vivo. LCCoA, long-chain fatty acyl-coenzyme A; mtGPAT, mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase; LPA, lysophosphatidic acid; AGPAT, acyl-CoA:1-acylglycerol-sn-3-phosphate acyltransferase; PA, phosphatidic acid; PAP, phosphatidic acid phosphatase; DAG, diacylglycerol; DGAT2; acyl-CoA:diacylglycerol acyltransferase 2; TG, triglyceride; PKCε, protein kinase Cε.
Fig 7
Hepatic PNPLA3 mRNA expression level positively correlated with hepatic triglyceride, DAG content, and insulin resistance in humans. (A,B) Correlation between hepatic triglyceride or DAG content and hepatic PNPLA3 mRNA expression in humans (n = 35). (C) Correlation between hepatic PNPLA3 mRNA expression and HOMA-IR (n = 35). Black dots are wildtype (148I) subjects, triangles are 148M variant heterozygous carriers, and squares are 148M variant homozygous carriers.
Similar articles
- Second-generation antisense oligonucleotides against β-catenin protect mice against diet-induced hepatic steatosis and hepatic and peripheral insulin resistance.
Popov VB, Jornayvaz FR, Akgul EO, Kanda S, Jurczak MJ, Zhang D, Abudukadier A, Majumdar SK, Guigni B, Petersen KF, Manchem VP, Bhanot S, Shulman GI, Samuel VT. Popov VB, et al. FASEB J. 2016 Mar;30(3):1207-17. doi: 10.1096/fj.15-271999. Epub 2015 Dec 7. FASEB J. 2016. PMID: 26644352 Free PMC article. - Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance.
Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Choi CS, et al. J Biol Chem. 2007 Aug 3;282(31):22678-88. doi: 10.1074/jbc.M704213200. Epub 2007 May 27. J Biol Chem. 2007. PMID: 17526931 - Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease.
Chen W, Chang B, Li L, Chan L. Chen W, et al. Hepatology. 2010 Sep;52(3):1134-42. doi: 10.1002/hep.23812. Hepatology. 2010. PMID: 20648554 Free PMC article. - Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein.
Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Romeo S, et al. Curr Opin Lipidol. 2010 Jun;21(3):247-52. doi: 10.1097/mol.0b013e328338ca61. Curr Opin Lipidol. 2010. PMID: 20480550 Review. - Hepatic triglyceride synthesis and nonalcoholic fatty liver disease.
Choi SS, Diehl AM. Choi SS, et al. Curr Opin Lipidol. 2008 Jun;19(3):295-300. doi: 10.1097/MOL.0b013e3282ff5e55. Curr Opin Lipidol. 2008. PMID: 18460922 Review.
Cited by
- Effect of PNPLA3 rs738409 variant (I148 M) on hepatic steatosis, necroinflammation, and fibrosis in Japanese patients with chronic hepatitis C.
Yasui K, Kawaguchi T, Shima T, Mitsuyoshi H, Seki K, Sendo R, Mizuno M, Itoh Y, Matsuda F, Okanoue T. Yasui K, et al. J Gastroenterol. 2015 Aug;50(8):887-93. doi: 10.1007/s00535-014-1018-z. Epub 2014 Nov 26. J Gastroenterol. 2015. PMID: 25543233 - PNPLA3 genetic variation in alcoholic steatosis and liver disease progression.
Stickel F, Hampe J, Trépo E, Datz C, Romeo S. Stickel F, et al. Hepatobiliary Surg Nutr. 2015 Jun;4(3):152-60. doi: 10.3978/j.issn.2304-3881.2014.11.04. Hepatobiliary Surg Nutr. 2015. PMID: 26151055 Free PMC article. Review. - Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-alcoholic Fatty Liver.
Feldman A, Eder SK, Felder TK, Kedenko L, Paulweber B, Stadlmayr A, Huber-Schönauer U, Niederseer D, Stickel F, Auer S, Haschke-Becher E, Patsch W, Datz C, Aigner E. Feldman A, et al. Am J Gastroenterol. 2017 Jan;112(1):102-110. doi: 10.1038/ajg.2016.318. Epub 2016 Aug 16. Am J Gastroenterol. 2017. PMID: 27527746 - Antisense oligonucleotide is a promising intervention for liver diseases.
Lu K, Fan Q, Zou X. Lu K, et al. Front Pharmacol. 2022 Dec 9;13:1061842. doi: 10.3389/fphar.2022.1061842. eCollection 2022. Front Pharmacol. 2022. PMID: 36569303 Free PMC article. Review. - Genome-wide analysis of hepatic lipid content in extreme obesity.
DiStefano JK, Kingsley C, Craig Wood G, Chu X, Argyropoulos G, Still CD, Doné SC, Legendre C, Tembe W, Gerhard GS. DiStefano JK, et al. Acta Diabetol. 2015 Apr;52(2):373-82. doi: 10.1007/s00592-014-0654-3. Epub 2014 Sep 23. Acta Diabetol. 2015. PMID: 25246029 Free PMC article.
References
- Cheung O, Sanyal AJ. Recent advances in nonalcoholic fatty liver disease. Curr Opin Gastroenterol. 2010;26:202–208. - PubMed
- Chang CY. Understanding the relationship between PNPLA3, NAFLD and insulin resistance: do ethnic differences bring more questions or more answers? Liver Int. 2011;31:1246–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 DK088231/DK/NIDDK NIH HHS/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R24 DK085638/DK/NIDDK NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- P30 DK-34989/DK/NIDDK NIH HHS/United States
- AG-23686/AG/NIA NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
- DK-40936/DK/NIDDK NIH HHS/United States
- R01 DK049230/DK/NIDDK NIH HHS/United States
- RR-024139/RR/NCRR NIH HHS/United States
- DK-085638/DK/NIDDK NIH HHS/United States
- P60 AG010469/AG/NIA NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous