The histone deacetylase SIRT2 stabilizes Myc oncoproteins - PubMed (original) (raw)
doi: 10.1038/cdd.2012.147. Epub 2012 Nov 23.
N Xu, A Malyukova, C J Scarlett, Y T Sun, X D Zhang, D Ling, S-P Su, C Nelson, D K Chang, J Koach, A E Tee, M Haber, M D Norris, C Toon, I Rooman, C Xue, B B Cheung, S Kumar, G M Marshall, A V Biankin, T Liu
Affiliations
- PMID: 23175188
- PMCID: PMC3569991
- DOI: 10.1038/cdd.2012.147
The histone deacetylase SIRT2 stabilizes Myc oncoproteins
P Y Liu et al. Cell Death Differ. 2013 Mar.
Abstract
Myc oncoproteins are commonly upregulated in human cancers of different organ origins, stabilized by Aurora A, degraded through ubiquitin-proteasome pathway-mediated proteolysis, and exert oncogenic effects by modulating gene and protein expression. Histone deacetylases are emerging as targets for cancer therapy. Here we demonstrated that the class III histone deacetylase SIRT2 was upregulated by N-Myc in neuroblastoma cells and by c-Myc in pancreatic cancer cells, and that SIRT2 enhanced N-Myc and c-Myc protein stability and promoted cancer cell proliferation. Affymetrix gene array studies revealed that the gene most significantly repressed by SIRT2 was the ubiquitin-protein ligase NEDD4. Consistent with this finding, SIRT2 repressed NEDD4 gene expression by directly binding to the NEDD4 gene core promoter and deacetylating histone H4 lysine 16. Importantly, NEDD4 directly bound to Myc oncoproteins and targeted Myc oncoproteins for ubiquitination and degradation, and small-molecule SIRT2 inhibitors reactivated NEDD4 gene expression, reduced N-Myc and c-Myc protein expression, and suppressed neuroblastoma and pancreatic cancer cell proliferation. Additionally, SIRT2 upregulated and small-molecule SIRT2 inhibitors decreased Aurora A expression. Our data reveal a novel pathway critical for Myc oncoprotein stability, and provide important evidences for potential application of SIRT2 inhibitors for the prevention and therapy of Myc-induced malignancies.
Figures
Figure 1
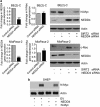
Upregulation of SIRT2 expression promotes neuroblastoma cell proliferation. (a and b) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, N-Myc siRNA-1, N-Myc siRNA-2, c-Myc siRNA-1, c-Myc siRNA-2, SIRT2 siRNA-1 or SIRT2 siRNA-2 for 48 h, followed by RNA and protein extraction, real-time RT-PCR (a) and immunoblot (b) analyses of N-Myc, c-Myc, and SIRT2 mRNA and protein expression. (c-e) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, N-Myc siRNA-1, N-Myc siRNA-2, c-Myc siRNA-1, c-Myc siRNA-2, SIRT2 siRNA-1 or SIRT2 siRNA-2 (c), or treated with vehicle control, the SIRT2-selective inhibitor AC-93253 (d) or the SIRT1/SIRT2 inhibitor Salermide (e). Seventy-two hours later, relative cell numbers were examined by Alamar blue assays, and expressed as percentage change in cell numbers. Error bars represented S.E. ***P<0.001
Figure 2
SIRT2 upregulates N-Myc and c-Myc protein expression by blocking their degradation. (a and b) BE(2)-C and MiaPaca-2 cells were treated with control, the SIRT2-selective inhibitor AC-93253 at 0.6 _μ_M or the SIRT1/SIRT2 inhibitor Salermide at 50 _μ_M, followed by real-time RT-PCR (a) and immunoblot (b) analyses of N-Myc and c-Myc mRNA and protein expression. Error bars represented standard error. **P<0.01 and P<0.001. (c) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, SIRT2 siRNA-1 or SIRT2 siRNA-2 for 48 h, followed by treatment with the proteasome inhibitor MG-132 (10 _μ_M) for 3 h. N-Myc and c-Myc protein expression was analyzed by immunoblot. (d) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA or SIRT2 siRNA-1 for 30 h, and treated with 50 _μ_M cycloheximide (CHX) for the last 0, 15, 30, 45, or 60 min in BE(2)-C cells and for the last 0, 10, 20, 30, or 45 min in MiaPaca-2 cells. Protein was extracted from the cells and subjected to immunoblot analysis of N-Myc and c-Myc. N-Myc and c-Myc protein levels were normalized by actin, the ratio of N-Myc protein/actin protein as well as the ratio of c-Myc protein/actin protein were artificially set as 1.0 for samples untreated with CHX, and half-life (T1/2) of N-Myc and c-Myc proteins was obtained from the line chart
Figure 3
SIRT2 stabilizes Myc proteins without modulating ERK protein phosphorylation and GSK3 protein expression. (a and b) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, SIRT2 siRNA-1 or SIRT2 siRNA-2, followed by protein extraction. (a) The expression of total N-Myc/c-Myc protein, N-Myc/c-Myc protein phosphorylated at S62 (S62-phos) and N-Myc/c-Myc protein phosphorylated at T58 (T58-phos) was analyzed by immunoblot with specific antibodies. (b) The expression of GSK3 protein, total ERK protein and phosphorylated ERK protein (phos-ERK) was analyzed by immunoblot with specific antibodies
Figure 4
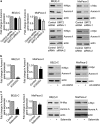
SIRT2 represses NEDD4 gene transcription by directly binding to NEDD4 gene promoter and repressing NEDD4 promoter activity. (a and b) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control, SIRT2 siRNA-1 or SIRT2 siRNA-2 (a), or treated with vehicle control or 0.6 _μ_M AC-93253 (b). NEDD4 mRNA and protein expression was analyzed by real-time RT-PCR and immunoblot. (c) ChIP assays were performed in BE(2)-C and MiaPaca-2 cells with a control, anti-SIRT2 or anti-acetyl H4K16 Ab, and real-time PCR with primers targeting control region (−2500 bp upstream of the NEDD4 gene transcription start site) or primers targeting part of the NEDD4 gene core promoter region (−394 to −263 bp upstream of the NEDD4 gene transcription start site). Fold enrichment of NEDD4 gene core promoter by control, anti-SIRT2 or anti-acetyl H4K16 Ab was calculated by dividing PCR products from primers targeting the NEDD4 gene core promoter by PCR products from primers targeting control region. Fold enrichment by control Ab was artificially set as 1.0. (d) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA or SIRT2 siRNA-2 for 48 h, followed by ChIP assays with an anti-acetyl H4K16 Ab and real-time PCR with primers targeting control region or primers targeting the NEDD4 gene core promoter region. Fold change in acetylated H4K16 at NEDD4 gene core promoter was obtained after dividing fold enrichment of acetylated H4K16 at NEDD4 gene core promoter in SIRT2 siRNA-2-transfected samples by fold enrichment of acetylated H4K16 at NEDD4 gene core promoter in control siRNA-transfected samples. (e) BE(2)-C cells were transfected with control siRNA or SIRT2 siRNA-2 for 24 h, followed by transfection with a wild-type or a mutant NEDD4 gene promoter construct for another 24 h. Luciferase activity was measured and expressed as relative luciferase activity (wild-type NEDD4 promoter construct/mutant NEDD4 promoter construct). Error bars represented standard error. *P<0.05
Figure 5
Repression of NEDD4 gene expression contributes to SIRT2-induced upregulation of Myc proteins. (a) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, NEDD4 siRNA or SIRT2 siRNA-1. NEDD4, N-Myc and c-Myc gene, and protein expression were analyzed by real-time RT-PCR and immunoblot. Error bars represented standard error. ***P<0.001. (b) SHEP neuroblastoma cells, which do not express N-Myc and c-Myc protein, were transfected with an empty vector, N-Myc-expressing construct and/or NEDD4-expressing construct. N-Myc protein expression was analyzed by immunoblot
Figure 6
NEDD4 protein directly binds to N-Myc protein and targets N-Myc protein for ubiquitination. (a) BE(2)-C cells were transfected with control siRNA, NEDD4 siRNA or SIRT2 siRNA for 48 h. Cytoplasmic and nuclear protein was separated and analyzed by immunoblot with anti-NEDD4, anti-SIRT2, anti-GAPDH (marker for cytoplasmic protein), and anti-E2F1 (marker for nuclear protein) antibodies. (b) HEK293 cells were transfected with constructs expressing empty vector, N-Myc and/or NEDD4. Protein from the cells was immunoprecipitated with an anti-N-Myc Ab or a control mouse Ab, and IPproducts were analyzed by immunoblot with an anti-NEDD4 Ab. (c) HEK293 cells were transfected with constructs expressing HA-ubiquitin, Flag-N-Myc and/or Flag-NEDD4. Upper panel: protein from the cells was immunoprecipitated with an anti-N-Myc Ab, and co-IP products were analyzed by immunoblot with an anti-HA Ab. Lower panel: 5% of whole-cell lysates was used as input and probed with an anti-Flag Ab. (d) HEK293 cells were transfected with constructs expressing Flag-N-Myc and Flag-NEDD4. Protein from the cells was precipitated with anti-Flag M2 beads and incubated with E1, a panel of E2 enzymes, HA-ubiquitin (HA-Ub), and ATP. Polyubiquitinated protein was detected by immunoblot (IB) with an anti-HA Ab (upper panel). The indicated smear appearing above 110 kDa represented polyubiquitinated NEDD4 and N-Myc (N-Myc-UBn), and the smear/ladder between 76 and 110 kDa represented N-Myc-UBn. Immunoblot results with an anti-Flag M2 Ab showing Flag-tagged products are shown in the lower panel. (e) HEK293 cells were transfected with constructs expressing Flag-N-Myc, Flag-NEDD4, mutant Flag-NEDD4, or empty vector. Protein from the cells was precipitated with anti-Flag M2 beads, and precipitated protein was incubated with E1, UbcH5a, HA-ubiquitin, and ATP, followed by immunoblot analysis with an anti-HA Ab
Figure 7
SIRT2 upregulates Aurora A expression. (a) BE(2)-C and MiaPaca-2 cells were transfected with scrambled control siRNA, SIRT2 siRNA-1 or SIRT2 siRNA-2 for 48 h, followed by real-time RT-PCR and immunoblot analyses of Aurora A mRNA and protein expression. (b and c) BE(2)-C and MiaPaca-2 cells were treated with vehicle control, the SIRT2 inhibitor AC-93253 at 0.8 _μ_M (b), or Salermide at 50 _μ_M (c) for 48 h, followed by real-time RT-PCR and immunoblot analyses of Aurora A mRNA and protein expression. Error bars represented standard error. *P<0.05
Similar articles
- Histone deacetylase 5 blocks neuroblastoma cell differentiation by interacting with N-Myc.
Sun Y, Liu PY, Scarlett CJ, Malyukova A, Liu B, Marshall GM, MacKenzie KL, Biankin AV, Liu T. Sun Y, et al. Oncogene. 2014 Jun 5;33(23):2987-94. doi: 10.1038/onc.2013.253. Epub 2013 Jul 1. Oncogene. 2014. PMID: 23812427 - Histone deacetylase 2 and N-Myc reduce p53 protein phosphorylation at serine 46 by repressing gene transcription of tumor protein 53-induced nuclear protein 1.
Shahbazi J, Scarlett CJ, Norris MD, Liu B, Haber M, Tee AE, Carrier A, Biankin AV, London WB, Marshall GM, Lock RB, Liu T. Shahbazi J, et al. Oncotarget. 2014 Jun 30;5(12):4257-68. doi: 10.18632/oncotarget.1991. Oncotarget. 2014. PMID: 24952595 Free PMC article. - Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma.
Otto T, Horn S, Brockmann M, Eilers U, Schüttrumpf L, Popov N, Kenney AM, Schulte JH, Beijersbergen R, Christiansen H, Berwanger B, Eilers M. Otto T, et al. Cancer Cell. 2009 Jan 6;15(1):67-78. doi: 10.1016/j.ccr.2008.12.005. Cancer Cell. 2009. PMID: 19111882 - Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions.
Yang B, Kumar S. Yang B, et al. Cell Death Differ. 2010 Jan;17(1):68-77. doi: 10.1038/cdd.2009.84. Cell Death Differ. 2010. PMID: 19557014 Free PMC article. Review. - When Just One Phosphate Is One Too Many: The Multifaceted Interplay between Myc and Kinases.
Boi D, Rubini E, Breccia S, Guarguaglini G, Paiardini A. Boi D, et al. Int J Mol Sci. 2023 Mar 1;24(5):4746. doi: 10.3390/ijms24054746. Int J Mol Sci. 2023. PMID: 36902175 Free PMC article. Review.
Cited by
- Histone Deacetylase Inhibitors for Peripheral T-Cell Lymphomas.
Irimia R, Piccaluga PP. Irimia R, et al. Cancers (Basel). 2024 Sep 30;16(19):3359. doi: 10.3390/cancers16193359. Cancers (Basel). 2024. PMID: 39409979 Free PMC article. Review. - Golgi-localized Ring Finger Protein 121 is necessary for MYCN-driven neuroblastoma tumorigenesis.
Cheung BB, Mittra R, Murray J, Wang Q, Seneviratne JA, Raipuria M, Wong IPL, Restuccia D, Gifford A, Salib A, Sutton S, Huang L, Ferdowsi PV, Tsang J, Sekyere E, Mayoh C, Luo L, Brown DL, Stow JL, Zhu S, Young RJ, Solomon BJ, Chappaz S, Kile B, Kueh A, Herold MJ, Hilton DJ, Liu T, Norris MD, Haber M, Carter DR, Parker MW, Marshall GM. Cheung BB, et al. Commun Biol. 2024 Oct 14;7(1):1322. doi: 10.1038/s42003-024-06899-8. Commun Biol. 2024. PMID: 39402275 Free PMC article. - Screening approach by a combination of computational and in vitro experiments: identification of fluvastatin sodium as a potential SIRT2 inhibitor.
Yang J, Wang H, Liu J, Ma E, Jin X, Li Y, Ma C. Yang J, et al. J Mol Model. 2024 May 27;30(6):188. doi: 10.1007/s00894-024-05988-z. J Mol Model. 2024. PMID: 38801625 - Sirt2 inhibition improves gut epithelial barrier integrity and protects mice from colitis.
Hou D, Yu T, Lu X, Hong JY, Yang M, Zi Y, Ho TT, Lin H. Hou D, et al. Proc Natl Acad Sci U S A. 2024 Apr 30;121(18):e2319833121. doi: 10.1073/pnas.2319833121. Epub 2024 Apr 22. Proc Natl Acad Sci U S A. 2024. PMID: 38648480 Free PMC article. - SIRT2 promotes base excision repair by transcriptionally activating OGG1 in an ATM/ATR-dependent manner.
Geng A, Sun J, Tang H, Yu Y, Wang X, Zhang J, Wang X, Sun X, Zhou X, Gao N, Tan R, Xu Z, Jiang Y, Mao Z. Geng A, et al. Nucleic Acids Res. 2024 May 22;52(9):5107-5120. doi: 10.1093/nar/gkae190. Nucleic Acids Res. 2024. PMID: 38554113 Free PMC article.
References
- Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
- Maris JM, Matthay KK. Molecular biology of neuroblastoma. J Clin Oncol. 1999;17:2264–2279. - PubMed
- Mahlamaki EH, Barlund M, Tanner M, Gorunova L, Hoglund M, Karhu R, et al. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–358. - PubMed
- Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. C-myc activation in primary and metastatic ductal adenocarcinoma of the pancreas: Incidence, mechanisms, and clinical significance. Mod Pathol. 2002;15:462–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources