MRI for attenuation correction in PET: methods and challenges - PubMed (original) (raw)
Review
MRI for attenuation correction in PET: methods and challenges
Gudrun Wagenknecht et al. MAGMA. 2013 Feb.
Abstract
In current combined PET/MR systems, PET attenuation correction is based on MRI, since the small bore inside MRI systems and the strong magnetic field do not permit a rotating PET transmission source or a CT device to be integrated. Unlike CT measurements in PET/CT scanners, the MR signal is not directly correlated to tissue density and thus cannot be converted by a simple transformation of intensity values. Various approaches have been developed based on templates, atlas information, direct segmentation of T1-weighted MR images, or segmentation of images from special MR sequences. The advantages and disadvantages of these approaches as well as additional challenges will be discussed in this review.
Figures
Fig. 1
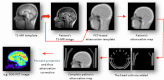
Hybrid PET/MR scanners: Philips Gemini TF PET/MR system where the PET and the MR system share a common patient table [1] (courtesy of H. Zaidi) (a); and Siemens mMR system where the PET detector is located between the gradient and the radiofrequency coils. (courtesy of R. Ladebeck and J. Georgi, Siemens Medical Solutions) (b)
Fig. 2
Steps in a template-based attenuation correction approach for brain [14]: The MR template is warped to match the individual MR image using SPM. The obtained transformation matrix is applied to warp the attenuation map template to generate an individualised attenuation map to which the coil attenuation map is added. The attenuation correction factors are obtained by forward projection
Fig. 3
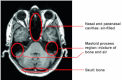
The T1-weighted MR image slice shows an air-filled nasal cavity, the mastoid process as a mixture of lamellar bone and small cavities, and the skull. The dark areas representing bone and air appear in the same intensity range
Fig. 4
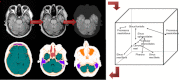
Principles of the direct knowledge-based segmentation approach for attenuation correction in brain studies presented in [31]: The input image (top left) is classified (top middle) and postprocessed to separate the extracerebral region (top right). The extracerebral region is segmented utilising class properties and relative positions of the regions (right). Segmented cavities and bone regions are the frontal sinus (mauve), the nasal cavity/ethmoidal cells/sphenoidal sinus (dark red), the maxillary sinuses (orange) and the pharynx (amber), the mastoid process (light blue), bone (light pink), brain (green) and CSF (dark green) tissue, and extracerebral soft tissue (white) (bottom)
Fig. 5
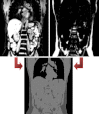
Dixon-based segmentation for whole-body attenuation correction shown in [44] (courtesy of A. Martinez-Moeller): MRI water (top left) and fat (top right) images acquired with a 2-point Dixon sequence are combined and segmented to generate the attenuation map for lungs, adipose tissue, soft tissue, and background
Fig. 6
In PET/MR, coils and other MR devices not visible in the MR images must be generated as template images, for example, in PET transmission scans and added to the attenuation map. Examples of a spine and a head coil (a) and a patient table (b) as well as one transaxial slice of the corresponding templates generated from PET transmission scans are shown [54] (courtesy of B. Zhang)
Fig. 7
The arms are not completely covered in the whole-body MR scan leading to truncation artefacts in the attenuation map image (top left), which were successfully compensated (bottom left). The corresponding reconstructed PET images are shown in the middle column. The relative percentage difference image (right) shows the greatest changes around the arms, and moderate changes inside the trunk with the highest changes observed at surfaces of anatomical structures [35] (courtesy of Z. Hu)
Similar articles
- Challenges and current methods for attenuation correction in PET/MR.
Keereman V, Mollet P, Berker Y, Schulz V, Vandenberghe S. Keereman V, et al. MAGMA. 2013 Feb;26(1):81-98. doi: 10.1007/s10334-012-0334-7. Epub 2012 Aug 9. MAGMA. 2013. PMID: 22875599 Review. - PET/MRI in the Presence of Metal Implants: Completion of the Attenuation Map from PET Emission Data.
Fuin N, Pedemonte S, Catalano OA, Izquierdo-Garcia D, Soricelli A, Salvatore M, Heberlein K, Hooker JM, Van Leemput K, Catana C. Fuin N, et al. J Nucl Med. 2017 May;58(5):840-845. doi: 10.2967/jnumed.116.183343. Epub 2017 Jan 26. J Nucl Med. 2017. PMID: 28126884 Free PMC article. - Simultaneous reconstruction of activity and attenuation for PET/MR.
Salomon A, Goedicke A, Schweizer B, Aach T, Schulz V. Salomon A, et al. IEEE Trans Med Imaging. 2011 Mar;30(3):804-13. doi: 10.1109/TMI.2010.2095464. Epub 2010 Nov 29. IEEE Trans Med Imaging. 2011. PMID: 21118768 - One registration multi-atlas-based pseudo-CT generation for attenuation correction in PET/MRI.
Arabi H, Zaidi H. Arabi H, et al. Eur J Nucl Med Mol Imaging. 2016 Oct;43(11):2021-35. doi: 10.1007/s00259-016-3422-5. Epub 2016 Jun 3. Eur J Nucl Med Mol Imaging. 2016. PMID: 27260522 - Whole-body simultaneous positron emission tomography (PET)-MR: optimization and adaptation of MRI sequences.
Fowler KJ, McConathy J, Narra VR. Fowler KJ, et al. J Magn Reson Imaging. 2014 Feb;39(2):259-68. doi: 10.1002/jmri.24308. Epub 2013 Oct 29. J Magn Reson Imaging. 2014. PMID: 24436150 Review.
Cited by
- DiCyc: GAN-based deformation invariant cross-domain information fusion for medical image synthesis.
Wang C, Yang G, Papanastasiou G, Tsaftaris SA, Newby DE, Gray C, Macnaught G, MacGillivray TJ. Wang C, et al. Inf Fusion. 2021 Mar;67:147-160. doi: 10.1016/j.inffus.2020.10.015. Inf Fusion. 2021. PMID: 33658909 Free PMC article. - Assessment of acute bone loading in humans using [18F]NaF PET/MRI.
Haddock B, Fan AP, Uhlrich SD, Jørgensen NR, Suetta C, Gold GE, Kogan F. Haddock B, et al. Eur J Nucl Med Mol Imaging. 2019 Nov;46(12):2452-2463. doi: 10.1007/s00259-019-04424-2. Epub 2019 Aug 5. Eur J Nucl Med Mol Imaging. 2019. PMID: 31385012 Free PMC article. - Continuous MR bone density measurement using water- and fat-suppressed projection imaging (WASPI) for PET attenuation correction in PET-MR.
Huang C, Ouyang J, Reese TG, Wu Y, El Fakhri G, Ackerman JL. Huang C, et al. Phys Med Biol. 2015 Oct 21;60(20):N369-81. doi: 10.1088/0031-9155/60/20/N369. Epub 2015 Sep 25. Phys Med Biol. 2015. PMID: 26405761 Free PMC article. - Attenuation correction in emission tomography using the emission data--A review.
Berker Y, Li Y. Berker Y, et al. Med Phys. 2016 Feb;43(2):807-32. doi: 10.1118/1.4938264. Med Phys. 2016. PMID: 26843243 Free PMC article. - Generating PET Attenuation Maps via Sim2Real Deep Learning-Based Tissue Composition Estimation Combined with MLACF.
Kobayashi T, Shigeki Y, Yamakawa Y, Tsutsumida Y, Mizuta T, Hanaoka K, Watanabe S, Morimoto-Ishikawa D, Yamada T, Kaida H, Ishii K. Kobayashi T, et al. J Imaging Inform Med. 2024 Feb;37(1):167-179. doi: 10.1007/s10278-023-00902-0. Epub 2024 Jan 10. J Imaging Inform Med. 2024. PMID: 38343219 Free PMC article.
References
- Herzog H, van den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q J Nucl Med Mol Imag. 2012;56:247–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical