CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis - PubMed (original) (raw)
CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis
Rachel E Miller et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Osteoarthritis is one of the leading causes of chronic pain, but almost nothing is known about the mechanisms and molecules that mediate osteoarthritis-associated joint pain. Consequently, treatment options remain inadequate and joint replacement is often inevitable. Here, we use a surgical mouse model that captures the long-term progression of knee osteoarthritis to longitudinally assess pain-related behaviors and concomitant changes in the innervating dorsal root ganglia (DRG). We demonstrate that monocyte chemoattractant protein (MCP)-1 (CCL2) and its high-affinity receptor, chemokine (C-C motif) receptor 2 (CCR2), are central to the development of pain associated with knee osteoarthritis. After destabilization of the medial meniscus, mice developed early-onset secondary mechanical allodynia that was maintained for 16 wk. MCP-1 and CCR2 mRNA, protein, and signaling activity were temporarily up-regulated in the innervating DRG at 8 wk after surgery. This result correlated with the presentation of movement-provoked pain behaviors, which were maintained up to 16 wk. Mice that lack Ccr2 also developed mechanical allodynia, but this started to resolve from 8 wk onwards. Despite severe allodynia and structural knee joint damage equal to wild-type mice, Ccr2-null mice did not develop movement-provoked pain behaviors at 8 wk. In wild-type mice, macrophages infiltrated the DRG by 8 wk and this was maintained through 16 wk after surgery. In contrast, macrophage infiltration was not observed in Ccr2-null mice. These observations suggest a key role for the MCP-1/CCR2 pathway in establishing osteoarthritis pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
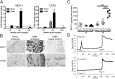
MCP-1/CCR2 gene and protein expression in DRG is elevated 8 wk after DMM surgery. (A) Real-time RT-PCR of Mcp-1 and Ccr2 in sham and DMM wild-type mice normalized to age-matched naïve levels: at 4 wk postsurgery, n = 5, two-tailed t test, P > 0.05; at 8 wk postsurgery, n = 4 for naïve, n = 5 for sham and DMM, one-way ANOVA with Bonferroni posttest, MCP-1: *P < 0.001 vs. sham and naïve, CCR2: *P < 0.001 vs. naïve, and P < 0.01 vs. sham; at 16 wk postsurgery, n = 4 for naïve, n = 7 for DMM, two-tailed t test *P = 0.01 for MCP-1 vs. naïve, *P = 0.003 for CCR2 vs. naïve. Results show mean ± SEM. (B) Representative images of in situ hybridization using antisense probes for MCP-1 and CCR2 in DRG sections (L3–L5) taken from DMM wild-type mice at 8 wk postsurgery and age-matched naïve mice. Sense probe control is shown for the DMM condition. Magnification 10×. (Scale bars, 100 μm.) (C) Protein levels of MCP-1 in the supernatants of cells cultured from age-matched naïve, sham, and DMM mice at 4 and 8 wk postsurgery, n = 6 wells, representative of two independent experiments, one-way ANOVA with Bonferroni posttest, ***P < 0.001. Results show mean ± 95% confidence interval. (D) Representative traces of individual cells during calcium mobilization assay indicating a response to MCP-1. Response to 50 mM potassium solution, used as a positive control, is also shown.
Fig. 2.
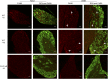
Increases in macrophage DRG populations are seen at 8 and 16 wk after DMM surgery in wild-type mice. Doublecortin (DCX, neuron, green) and F4/80 (macrophage, red) staining of ipsilateral DRG in age-matched naïve and DMM wild-type (WT) mice at 8 wk postsurgery (Top), age-matched naïve and DMM wild-type mice at 16 wk postsurgery (Middle), and age-matched naïve and DMM _Ccr2_-null mice at 8 wk postsurgery (Bottom). White arrows indicate example macrophage staining. Magnification 20×. (Scale bars, 50 μm.)
Fig. 3.
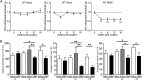
Pain-related behaviors develop progressively in wild-type mice after DMM surgery. (A) Mechanical allodynia in the ipsilateral hindpaw of naïve (n = 7–10), sham (n = 9), and DMM mice (n = 9–13), Naïve time 0: n = 30, one-way ANOVA with Bonferroni’s multiple comparison test, **P < 0.01, ***_P_ < 0.001 vs. Time 0. Results show mean ± SEM. (_B_) Distance traveled, average number of climbs per hour, and average number of rears per hour during a 15-h period on a LABORAS platform. At 4 wk, _n_ = 8 naïve, _n_ = 10 DMM, _P_ > 0.05 by two-tailed t test. At 8 wk, n = 18 naïve, n = 10 sham, n = 15 DMM, *P < 0.05 and **P < 0.01 by one-way ANOVA with Bonferroni’s multiple comparison test. At 16 wk, n = 9 naïve and n = 8 DMM, *P = 0.03 for distance, **P = 0.003 for climbing, and *P = 0.0487 for rearing by two-tailed t test. For LABORAS results, data were log-transformed if necessary to ensure normality as determined by the D’Agostino–Pearson normality test before analysis. Results show mean ± SEM.
Fig. 4.
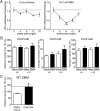
_Ccr2_-null mice present different pain-related behaviors after DMM surgery. (A) Mechanical allodynia in _Ccr2_-null naïve (n = 6–11) and DMM mice (n = 5–10), Naïve time 0: n = 17, one-way ANOVA with Bonferroni’s multiple comparison test, *P < 0.05, **_P_ < 0.01, ***_P_ < 0.001 vs. Time 0, Mean ± SEM. (_B_) Distance traveled, average number of climbs per hour, and average number of rears per hour during a 15-h period. At 8 wk, naïve _n_ = 6, DMM _n_ = 9, _P_ > 0.05 by two-tailed t test. At 16 wk, naïve n = 11, DMM n = 6, P > 0.05 by two-tailed t test. (C) Distance traveled during a 15-h period after administration of CCR2 receptor antagonist (CCR2 RA) (5 mg/kg, i.p.) or DMSO vehicle control to wild-type DMM mice at 9 wk postsurgery; n = 5, *P = 0.0109 vs. DMSO control by two-tailed t test. Dashed line indicates wild-type naïve level. For LABORAS results, data were log-transformed if necessary to ensure normality as determined by the D’Agostino–Pearson normality test before analysis. Results show mean ± SEM.
Similar articles
- The role of intra-articular neuronal CCR2 receptors in knee joint pain associated with experimental osteoarthritis in mice.
Ishihara S, Obeidat AM, Wokosin DL, Ren D, Miller RJ, Malfait AM, Miller RE. Ishihara S, et al. Arthritis Res Ther. 2021 Apr 7;23(1):103. doi: 10.1186/s13075-021-02486-y. Arthritis Res Ther. 2021. PMID: 33827672 Free PMC article. - Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation.
Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ, Gao YJ. Zhu X, et al. J Pain. 2014 May;15(5):516-26. doi: 10.1016/j.jpain.2014.01.492. Epub 2014 Jan 23. J Pain. 2014. PMID: 24462503 - CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis.
Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH. Raghu H, et al. Ann Rheum Dis. 2017 May;76(5):914-922. doi: 10.1136/annrheumdis-2016-210426. Epub 2016 Dec 13. Ann Rheum Dis. 2017. PMID: 27965260 Free PMC article. - Chemokine signaling and the management of neuropathic pain.
White FA, Feldman P, Miller RJ. White FA, et al. Mol Interv. 2009 Aug;9(4):188-95. doi: 10.1124/mi.9.4.7. Mol Interv. 2009. PMID: 19720751 Free PMC article. Review. - Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway.
Kolattukudy PE, Niu J. Kolattukudy PE, et al. Circ Res. 2012 Jan 6;110(1):174-89. doi: 10.1161/CIRCRESAHA.111.243212. Circ Res. 2012. PMID: 22223213 Free PMC article. Review.
Cited by
- Post-traumatic osteoarthritis: from mouse models to clinical trials.
Little CB, Hunter DJ. Little CB, et al. Nat Rev Rheumatol. 2013 Aug;9(8):485-97. doi: 10.1038/nrrheum.2013.72. Epub 2013 May 21. Nat Rev Rheumatol. 2013. PMID: 23689231 Review. - IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis.
Faust HJ, Zhang H, Han J, Wolf MT, Jeon OH, Sadtler K, Peña AN, Chung L, Maestas DR Jr, Tam AJ, Pardoll DM, Campisi J, Housseau F, Zhou D, Bingham CO 3rd, Elisseeff JH. Faust HJ, et al. J Clin Invest. 2020 Oct 1;130(10):5493-5507. doi: 10.1172/JCI134091. J Clin Invest. 2020. PMID: 32955487 Free PMC article. - Identification and characterization of two consistent osteoarthritis subtypes by transcriptome and clinical data integration.
Coutinho de Almeida R, Mahfouz A, Mei H, Houtman E, den Hollander W, Soul J, Suchiman E, Lakenberg N, Meessen J, Huetink K, Nelissen RGHH, Ramos YFM, Reinders M, Meulenbelt I. Coutinho de Almeida R, et al. Rheumatology (Oxford). 2021 Mar 2;60(3):1166-1175. doi: 10.1093/rheumatology/keaa391. Rheumatology (Oxford). 2021. PMID: 32885253 Free PMC article. - Mitochondrial Transplantation Ameliorates the Development and Progression of Osteoarthritis.
Lee AR, Woo JS, Lee SY, Na HS, Cho KH, Lee YS, Lee JS, Kim SA, Park SH, Kim SJ, Cho ML. Lee AR, et al. Immune Netw. 2022 Jan 21;22(2):e14. doi: 10.4110/in.2022.22.e14. eCollection 2022 Apr. Immune Netw. 2022. PMID: 35573148 Free PMC article. - Osteoarthritis pain mechanisms: basic studies in animal models.
Zhang RX, Ren K, Dubner R. Zhang RX, et al. Osteoarthritis Cartilage. 2013 Sep;21(9):1308-15. doi: 10.1016/j.joca.2013.06.013. Osteoarthritis Cartilage. 2013. PMID: 23973145 Free PMC article. Review.
References
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–973. - PubMed
- Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–3553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR060364/AR/NIAMS NIH HHS/United States
- T32 AR007590/AR/NIAMS NIH HHS/United States
- R01AR060364/AR/NIAMS NIH HHS/United States
- 5T32AR007590-15/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous