Fluorescent probes and fluorescence (microscopy) techniques--illuminating biological and biomedical research - PubMed (original) (raw)
Review
Fluorescent probes and fluorescence (microscopy) techniques--illuminating biological and biomedical research
Gregor P C Drummen. Molecules. 2012.
Abstract
Fluorescence, the absorption and re-emission of photons with longer wavelengths, is one of those amazing phenomena of Nature. Its discovery and utilization had, and still has, a major impact on biological and biomedical research, since it enables researchers not just to visualize normal physiological processes with high temporal and spatial resolution, to detect multiple signals concomitantly, to track single molecules in vivo, to replace radioactive assays when possible, but also to shed light on many pathobiological processes underpinning disease states, which would otherwise not be possible. Compounds that exhibit fluorescence are commonly called fluorochromes or fluorophores and one of these fluorescent molecules in particular has significantly enabled life science research to gain new insights in virtually all its sub-disciplines: Green Fluorescent Protein. Because fluorescent proteins are synthesized in vivo, integration of fluorescent detection methods into the biological system via genetic techniques now became feasible. Currently fluorescent proteins are available that virtually span the whole electromagnetic spectrum. Concomitantly, fluorescence imaging techniques were developed, and often progress in one field fueled innovation in the other. Impressively, the properties of fluorescence were utilized to develop new assays and imaging modalities, ranging from energy transfer to image molecular interactions to imaging beyond the diffraction limit with super-resolution microscopy. Here, an overview is provided of recent developments in both fluorescence imaging and fluorochrome engineering, which together constitute the “fluorescence toolbox” in life science research.
Figures
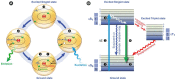
Figure 1
Luminescence in Nature. (A) Sepiolidae family of squid; (B) Aurelia aurita, moon jelly fish; please note that the blue glow stems from diffraction and not from bioluminescence; (C) Lampyridae family of fireflies; (D) Phosphorescent zinc sulfide pigment (alkaline earth metal); (E) Fluorescent rocks (top is illuminated with UV-light; bottom white light recording): left = Willemite/Calcite; right = Hardystonite (courtesy Ron Teunissen ©2012); (F) Luminescent water at the Gippsland Lakes, Australia (courtesy Phil Hart ©2012), which is created by Noctiluca scintillans, a non-parasitic marine-dwelling species of dinoflagellate that exhibits bioluminescence; the bioluminescent reaction is instantaneous, as observed in the left picture when the water is mechanically disturbed; (G) Mycena Chlorophos, a large genus of small saprotrophic mushrooms.
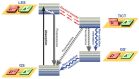
Figure 2
Fluorescence principle. (A) Schematic representation of the fluorescence phenomenon in the classical Bohr model. From the ground state _GS_0❶, absorption of a light quantum (blue) causes an electron to move to a higher energy level ❷→❸. After residing in this “excited state” ❸ for a particular time, the fluorescence lifetime, the electron falls back to its original level ❸→❹ and the fluorochrome dissipates the excess energy by emitting a photon (green) ❹. (B) Jabłoński diagram: Upon photon absorption, a ground state _GS_0 electron (electronic singlet) is promoted to a higher and excited state, relaxes quickly to a lower vibrational excited state (white line) and thereby looses energy. When returning to the ground state, it dissipates the remaining energy by emitting a photon with a longer wavelength, i.e., fluorescence emission. The spins of electrons in the singlet states (paired or unpaired anti-parallel spins) compared to the triplet state (unpaired, parallel spin) are depicted. Notice that intersystem crossing from _ES_1→ _ET_1 requires spin conversion and phosphorescence occurs through relaxation from the triplet excited state.
Figure 3
Twisted Intramolecular Charge Transfer (TICT) dynamics. Upon excitation from the ground state (GS), the locally excited state (LES) equilibrates rapidly with the TICT state after fast electron transfer. The TICT state is energetically lower and relaxation from the TICT state occurs either radiatively or non-radiatively to a thermally hot ground state (GS’) which after dissipation of heat becomes the GS. Alternatively, excess energy is dissipated via direct radiative relaxation to the GS. The exact pathway for energy dissipation depends strongly on the polarity of the environment.
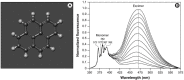
Figure 4
Pyrene’s excimer formation in lipid vesicles. (A) Molecular structure of pyrene (Benzo[d,e,f]phenanthrene; C16H10). (B) Emission spectra of pyrene in egg-PC. Spectra are normalized to the 372 nm peak of the monomer and excimer formation can increasingly be observed at ~460 nm with increasing pyrene concentration.
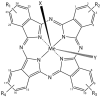
Figure 5
General chemical structure of metal-phthalocyanine dyes. Me denotes the metal ion; R1-4 are peripheral functional groups and X and Y are optional functional groups above and below the molecule’s plane (axial).
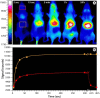
Figure 6
Bio-imaging with hyaluronan analogs modified with the near-infrared IR-783 dye.(A) Optical images of SKH mice injected with fluorescent HA-NIRdye (1% dye load). Based on Reference [76]. Note that the probe predominantly accumulates in the upper abdominal and thoracic organs, i.e., liver, heart, lungs, kidneys, stomach, and lymph nodes. After 24−48 h, the probe is exclusively found in the liver. (B) Progression of the fluorescence intensity in the boxes indicated in A.
Similar articles
- Fluorescent probes for super-resolution imaging in living cells.
Fernández-Suárez M, Ting AY. Fernández-Suárez M, et al. Nat Rev Mol Cell Biol. 2008 Dec;9(12):929-43. doi: 10.1038/nrm2531. Epub 2008 Nov 12. Nat Rev Mol Cell Biol. 2008. PMID: 19002208 Review. - Multicolor conjugated polymer dots for biological fluorescence imaging.
Wu C, Bull B, Szymanski C, Christensen K, McNeill J. Wu C, et al. ACS Nano. 2008 Nov 25;2(11):2415-23. doi: 10.1021/nn800590n. ACS Nano. 2008. PMID: 19206410 Free PMC article. - Far-red organic fluorophores contain a fluorescent impurity.
Stone MB, Veatch SL. Stone MB, et al. Chemphyschem. 2014 Aug 4;15(11):2240-6. doi: 10.1002/cphc.201402002. Epub 2014 Apr 29. Chemphyschem. 2014. PMID: 24782148 Free PMC article. - Photostable and photoswitching fluorescent dyes for super-resolution imaging.
Minoshima M, Kikuchi K. Minoshima M, et al. J Biol Inorg Chem. 2017 Jul;22(5):639-652. doi: 10.1007/s00775-016-1435-y. Epub 2017 Jan 12. J Biol Inorg Chem. 2017. PMID: 28083655 Review. - Resolution of multiple green fluorescent protein color variants and dyes using two-photon microscopy and imaging spectroscopy.
Lansford R, Bearman G, Fraser SE. Lansford R, et al. J Biomed Opt. 2001 Jul;6(3):311-8. doi: 10.1117/1.1383780. J Biomed Opt. 2001. PMID: 11516321
Cited by
- Red-Emitting Dithienothiophene S,_S_-Dioxide Dyes for Cellular Membrane Staining.
Rzewnicka A, Krysiak J, Pawłowska R, Żurawiński R. Rzewnicka A, et al. Materials (Basel). 2023 Feb 22;16(5):1806. doi: 10.3390/ma16051806. Materials (Basel). 2023. PMID: 36902920 Free PMC article. - Synthesis, fluorescence properties and the promising cytotoxicity of pyrene-derived aminophosphonates.
Lewkowski J, Rodriguez Moya M, Wrona-Piotrowicz A, Zakrzewski J, Kontek R, Gajek G. Lewkowski J, et al. Beilstein J Org Chem. 2016 Jun 16;12:1229-35. doi: 10.3762/bjoc.12.117. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27559373 Free PMC article. - Non-Phenomenological Description of the Time-Resolved Emission in Solution with Quantum-Classical Vibronic Approaches-Application to Coumarin C153 in Methanol.
Cerezo J, Gao S, Armaroli N, Ingrosso F, Prampolini G, Santoro F, Ventura B, Pastore M. Cerezo J, et al. Molecules. 2023 May 5;28(9):3910. doi: 10.3390/molecules28093910. Molecules. 2023. PMID: 37175320 Free PMC article. - Hypoxia-activated probe for NIR fluorescence and photoacoustic dual-mode tumor imaging.
Li M, Li H, Wu Q, Niu N, Huang J, Zhang L, Li Y, Wang D, Tang BZ. Li M, et al. iScience. 2021 Mar 2;24(3):102261. doi: 10.1016/j.isci.2021.102261. eCollection 2021 Mar 19. iScience. 2021. PMID: 33763638 Free PMC article. - Fluorescence Imaging of Mitochondria with Three Different Sets of Signals Based on Fluorene Cation Fluorescent Probe.
Wang W, Liu Y, Niu J, Lin W. Wang W, et al. J Fluoresc. 2019 Nov;29(6):1457-1465. doi: 10.1007/s10895-019-02451-8. Epub 2019 Nov 26. J Fluoresc. 2019. PMID: 31773380
References
- Chalfie M., Tu Y., Euskirchen G., Ward W.W., Prasher D.C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources