Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung - PubMed (original) (raw)
. 2013 Jan 15;373(2):267-80.
doi: 10.1016/j.ydbio.2012.11.012. Epub 2012 Nov 27.
Carsten Schnatwinkel, Lee Hazelwood, Lauren Chessum, Anju Paudyal, Helen Hilton, M Rosario Romero, Jonathan Wilde, Debora Bogani, Jeremy Sanderson, Caroline Formstone, Jennifer N Murdoch, Lee A Niswander, Andy Greenfield, Charlotte H Dean
Affiliations
- PMID: 23195221
- PMCID: PMC3549499
- DOI: 10.1016/j.ydbio.2012.11.012
Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung
Laura L Yates et al. Dev Biol. 2013.
Abstract
During lung development, proper epithelial cell arrangements are critical for the formation of an arborized network of tubes. Each tube requires a lumen, the diameter of which must be tightly regulated to enable optimal lung function. Lung branching and lumen morphogenesis require close epithelial cell-cell contacts that are maintained as a result of adherens junctions, tight junctions and by intact apical-basal (A/B) polarity. However, the molecular mechanisms that maintain epithelial cohesion and lumen diameter in the mammalian lung are unknown. Here we show that Scribble, a protein implicated in planar cell polarity (PCP) signalling, is necessary for normal lung morphogenesis. Lungs of the Scrib mouse mutant Circletail (Crc) are abnormally shaped with fewer airways, and these airways often lack a visible, 'open' lumen. Mechanistically we show that Scrib genetically interacts with the core PCP gene Vangl2 in the developing lung and that the distribution of PCP pathway proteins and Rho mediated cytoskeletal modification is perturbed in Scrib(Crc/Crc) lungs. However A/B polarity, which is disrupted in Drosophila Scrib mutants, is largely unaffected. Notably, we find that Scrib mediates functions not attributed to other PCP proteins in the lung. Specifically, Scrib localises to both adherens and tight junctions of lung epithelia and knockdown of Scrib in lung explants and organotypic cultures leads to reduced cohesion of lung epithelial cells. Live imaging of Scrib knockdown lungs shows that Scrib does not affect bud bifurcation, as previously shown for the PCP protein Celsr1, but is required to maintain epithelial cohesion. To understand the mechanism leading to reduced cell-cell association, we show that Scrib associates with β-catenin in embryonic lung and the sub-cellular distribution of adherens and tight junction proteins is perturbed in mutant lung epithelia. Our data reveal that Scrib is required for normal lung epithelial organisation and lumen morphogenesis by maintaining cell-cell contacts. Thus we reveal novel and important roles for Scrib in lung development operating via the PCP pathway, and in regulating junctional complexes and cell cohesion.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Fig. S1
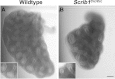
Confocal DIC images of E14.5 wildtype and Scrib1 Crc/Crc left lung lobes. Confocal DIC images of E14.5 wildtype (A) and Scrib1 Crc/Crc (B) left lung lobes. Homozygous mutant lungs are smaller with fewer branches and narrower lumina (see insets for detail).
Fig. S2
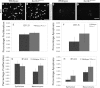
Proliferation and apoptosis are unaffected in Scrib1 Crc/Crc lungs. E14.5 wildtype ((A) and (C)) and Scrib1 Crc/Crc ((B) and (D)) sections immunostained with anti-phosphohistone-3 (PH3) ((A) and (B)) to assess proliferation or anti-cleaved caspase-3 ((C) and (D)) to assess apoptosis. Quantification at both E11.5 ((E) and (F)) and E14.5 ((G) and (H)) shows no difference in the percentage of proliferating cells ((E) and (G)) or the percentage of cells undergoing programmed cell death ((F) and (H)) between Scrib1 Crc/Crc and wildtype littermates. (E) wildtype _mean_=4.8%±0.38, _n_=9, _Crc mean_=5.2%±0.34, _n_=9. (F), wildtype _mean_=1.38%, ±0.63, _n_=8, Crc _mean_=2.50%±1.18, _n_=6. (G), Wildtype epithelium mean=3.40%, ±0.54, _n_=16, Crc epithelium mean=3.04%, ±0.41, _n_=21; wildtype mesenchyme mean=2.11%, ±0.28, _n_=8, Crc mesenchyme mean=3.05%, ±0.32, _n_=14. (H), wildtype epithelium mean=0.05%, ±0.05, _n_=8, Crc epithelium mean=0.17%, ±0.10, _n_=6; wildtype mesenchyme mean=2.71%, ±0.15, _n_=7, Crc mesenchyme mean=0.42%, ±0.16, _n_=6 Scale bars; A–D 12.5 μM.
Fig. S3
Differentiation is unaffected in Scrib1 Crc/Crc lungs. Analysis of two proximal airway markers, αSMA ((A) and (B)) and Clara cell-10 ((C) and (D)), and two distal airway markers, Pro-SpC ((E) and(F)) and Aqp5 ((G) and (H)), by immunohistochemistry revealed no obvious differences in differentiation of Scrib1 Crc/Crc lungs ((B), (D), (F) and (H)) compared to wildtype ((A), (C), (E) and (G)). Western blotting revealed the levels of both total and phospho-aPKC remained similar in Scrib1 Crc/Crc lungs compared to wildtype littermates (I). Scale bars; A–H 12.5 μM.
Fig. S4
Scrib1 shows genetic interaction with Vangl2. E14.5 paraffin sections from wildtype littermates (A _n_=3), Scrib1 Crc/+ (B _n_=2), Vangl2 Lp/+ (C _n_=3) or Scrib1 Crc/+; Vangl2 Lp/+ double heterozygotes (D _n_=4) were stained with H&E to compare histology. Airways in Scrib1 Cr//+(B) and Vangl2 Lp/+ (C) heterozygous lung sections show open organised airways, similar to those seen in wildtype sections (A) although minor epithelial disorganisation and slight narrowing of lumina is observed in Vangl2 Lp/+. In contrast, the lungs of compound heterozygotes (D) contain disorganised airways with severely reduced or absent lumina. Insets in A and D show a single epithelial airway at high magnification. Scale bars; A and B 25 μM, insets 12.5 μM.
Fig. S5
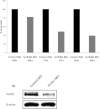
Validation of morpholino knockdown. E11.5 lungs were cultured for between 48 and 72 or 96 h in the presence of Scrib1 or control morpholinos (MO) and then protein extracted for Western blotting with anti-Scrib1 antibodies; _n_=3 explants (minimum) for each condition at each timepoint. Quantification of relative protein levels following Western blotting revealed an increasing reduction in Scrib1 protein following Scrib1 morpholino treatment over time. IB, immunoblotting shows an example of data from 96 h culture.
Fig. S6
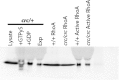
Western blot showing comparison of total RhoA and active RhoA in wilddtype and Scrib1 Crc/Crc. Raw data of image shown in Fig. 3A.
Fig. 1
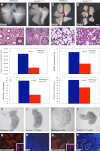
Scrib1 is required for normal lung development. E14.5 ((A) and (B)) and E18.5 ((C) and (D)) wildtype ((A) and, (C)) and Scrib1 Crc/Crc lungs ((B) and, (D)) show mutant lung lobes are smaller and misshapen. Histological analysis of E14.5H&E stained sections ((E) and (F)) shows fewer, less organised epithelial airways in Scrib1 Crc/Crc lungs ((F), (K): wildtype mean=44.42 μM, ±2.43, Crc mean=17.17 μM, ±1.64), with reduced lumen area ((I): wildtype mean=28.2%, ±2.93, _n_=39, Crc mean=9.50% ±1.12, _n_=35), compared with wildtype ((E), (I) and (K)). Quantification of airway width highlights the reduced diameter of Scrib1 Crc/Crc lumina ((J): Crc mean=5.94 μM, ±0.05 compared to wildtype mean=12.13 μM, ±0.05). By E18.5 severe hypoplasia is evident, with significant reduction in airway number in Scrib1 Crc/Crc lungs ((H), (L): mean=63.33 μM, ±1.61) compared to wildtype ((G), (L): mean=90.89 μM, ±3.97). E11.5 left lung lobes from wildtype (M) and Scrib1 Crc/Crc (N) with identical numbers of buds at _t_=0 were cultured ex vivo. After 48 h Scrib1 Crc/Crc lungs had developed far fewer new buds (P) than wildtype lungs (O); representative images for each timepoint are shown. Immunostaining of E14.5 transverse cryosections with anti-pan-cytokeratin ((Q) and (S)) highlights disorganisation of epithelial cells in Scrib1 Crc/Crc lungs (S) compared to controls (Q). Epithelial cells are difficult to distinguish by DAPI staining in Scrib1 Crc/Crc (T) but are easily distinguished in wildtype (R). Scale bars; A–D 62.5 μM, E–H 25 μM, M–P 63 μM, Q–T 25 μM, insets in E, F 5 μM; *P<0.05.
Fig. 2
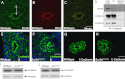
Apical–basal polarity appears unaffected but the cytoskeleton is disrupted in Scrib1 Crc/Crc lungs. Immunostaining of E11.5 transverse lung cryosections revealed no obvious defects in A/B polarity in Scrib1 Crc/Crc airways. aPKCς, is present at the apical surface in both Scrib1 Crc/Crc (red (B) and (D)) and wildtype sections (red (A) and (C)). Laminin localises to the basal side of the airways in both wildtype and mutant (green (A) and (B)). The Golgi marker GM130 (green (C) and (D)) is present on the apical side of DAPI-stained (blue (C) and (D)) nuclei in both Scrib1 Crc/Crc (D) and wildtype (C) lung sections. T.E.M. analysis of E16.5 lung revealed no apparent disruption to A/B polarity; in Scrib1 Crc/Crc (F) and wildtype controls (E), airway epithelial cells were orientated with their apical surface (recognised by the presence of microvilli) towards the lumen and apparently intact tight junctions (arrowheads). (TJ), tight junction. E14.5 ((G) and (H)) and E18.5 ((I) and (J)) Phalloidin-stained cryosections reveal severe disruption to the actin cytoskeleton in Scrib1 Crc/Crc ((H) and (J)) lungs; cortical actin was discontinuous and frequently not visible. In wildtype littermates, cortical actin was visible around the cell membranes (G, I). Immunostaining for non-muscle myosin IIA (K, L) also revealed disrupted distribution in Scrib1 Crc/Crc (L) lungs compared to wildtype (K). A–D 125 μM plus ×3 zoom, E–F 5 μM, G–J 5 μM K and L 25 μM.
Fig. 3
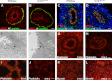
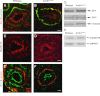
Active RhoA is reduced in Scrib1 Crc/Crc lungs and Scrib1 is required for correct localisation of Celsr1 and Vangl2 in lung epithelium. Western blotting (A) reveals a 60% reduction in the relative levels of active RhoA in E13.5 Scrib1 Crc/Crc lungs compared to wildtype littermates following active Rho pull-down and detection with RhoA (21KDa) antibody; (B) relative levels in wildtype: 100; Scrib1 Crc/Crc: 39.6, _p_=0.01, _n_=4 for each genotype. No alteration in levels of total RhoA was observed between Scrib1 Crc/Crc and wildtype (A). Levels of total and RhoA GTP were normalised to loading control levels detected with β-tubulin (50 kDa). E14.5 wildtype transverse lung cryosections immunostained with anti-Celsr1 shows localisation to epithelial cell membranes, at the apical and particularly around the basal side of airways in the basement membrane (C). In Scrib1 Crc/Crc sections, the basement membrane localisation is lost (D). In the Celsr1 Crsh/Crsh mutant, Scrib1 is weaker around epithelial membranes and appears more diffuse (E, compare to Scrib1 localization in wildtype lung I). At E14.5 Scrib1 is localised to the plasma membrane of epithelial and mesenchymal cells and is highly enriched towards the apical surface of airways (I). Scrib1 is absent in E14.5 Scrib1 Crc/Crc lungs (J). Punctate Vangl2 immunostaining is enriched towards the apical surface in wildtype sections (F) but this enrichment is less prominent in Scrib1 Crc/Crc (G). In Vangl2 Lp/Lp airways, Scrib1 immunostaining is unaffected (H). Scale bars; C–G, J 125 μM plus ×3 zoom, H 125 μM plus 2.5 zoom, I 125 μM plus 2 zoom.
Fig. 4
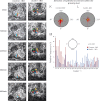
Morpholino knock-down of Scrib1 leads to misalignment of epithelial cells in ex vivo lung culture. E11.5 lung explants from β-actin promoter driven GFP embryos were cultured for 48 h with control or Scrib1 morpholinos and subsequently imaged over a period of 24 h (see Movies 1 and 2). During this 24 h time period, Scrib protein levels reduce from 80% of wildtype to 50%. Five time points from this series are depicted. Selected cells from control (A–E) or Scrib1 MO (F–J) treated explants were highlighted with different colours and their movements tracked over time. Epithelial cells from control MO treatment maintain a close neighbor–neighbor relationship, whereas with Scrib1 MO treatment, the cells are more loosely associated and often change their position (in particular, compare the relationship between the cells marked in red and green and in purple and blue and in purple and orange). The distribution of the direction of epithelial cell movements revealed by tracking ∼150 cells within the distal epithelium shows a close to random pattern in _Scrib1_-MO treated lung explants (L) compared to control (K) which shows more directional cell movements. Graphical representation showing the frequency with which cells migrate at each angle (M). P<0.0001, Mann–Whitney U test.
Fig. 5
Scrib1 is localised to the cell membrane and tight junctions and interacts with β-catenin. Transverse cryosection of E11.5 ((A)–(C)) wildtype lung immunostained for Scrib1 ((A) and (C)) shows Scrib1 is localised to membranes of epithelial and mesenchymal cells; strong staining is also observed apically, where Scrib1 co-localises with ZO-2 ((B) and (C)). Immunoprecipitation with β-Catenin antibody revealed an interaction with Scrib1 in endogenous lung tissue, suggesting these proteins physically interact in the lung (D). A thin band of β-catenin is observed around the basolateral membranes of E14.5 wildtype airways and is predominant at the apical membrane (E). In Scrib1 Crc/Crc airways, β-catenin appears diffuse and unevenly distributed around cells (F). E-cadherin distribution is largely unaltered in Scrib1 Crc/Crc airways (G) compared to controls (H). Western blotting shows no significant difference in the quantity of β-catenin (95 kDa) (I) or E-cadherin (120 kDa) protein (J) between E14.5 wildtype and Scrib1 Crc/Crc whole lung. Scale bars; A–C 125 μM plus ×2 zoom, E, F, G and H 125 μM plus ×3 zoom a, apical, b, basal.
Fig. 6
Specific tight junction proteins are disrupted in Scrib1 Crc/Crc lungs. E14.5 transverse wildtype ((A), (C) and (E)) and Scrib1 Crc/Crc ((B), (D) and (F)) lung sections immunostained for tight junction markers. Apical localisation of ZO-2 is maintained in Scrib1 Crc/Crc lungs (B), but its localisation is abnormal compared to wildtype (A). Claudin-18 is also mislocalised in Scrib1 Crc/Crc epithelial airways (D) compared to wildtype (C). ZO-1 appears normal in Scrib1 Crc/Crc lungs ((E) and (F)). ((G) and (H)) Levels of ZO-1 (220 kDa), ZO-2 (160 kDa) (G) and Claudin-18 (23 kDa) (H) were similar in wildtype and Scrib1 Crc/Crc lungs by Western blot. Scale bars; A–F 125 μM plus ×3 zoom.
Fig. 7
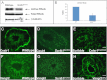
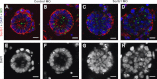
Scrib1 knockdown prevents normal epithelial cyst formation in organotypic cultures. Morpholino (MO, A–D, green) knockdown of Scrib1 ((C) and (D)) in organotypic cultures inhibits re-association of epithelial cells into organized cysts with a centrally located lumen. Scrib1 ((A)–(D), red) and DAPI ((A)–(H), blue/white) staining reveal organised epithelial cells surrounding a centrally located lumen in cultures containing control MO ((A), (B), (E) and (F)). In the presence of Scrib1 MO, cysts are comprised of misaligned epithelial cells and either a small non-central lumen or, frequently no visible lumen ((C), (D), (G) and (H)). Scale bars; A–H 125 μM ×2 zoom.
Fig. 8
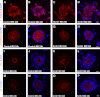
Scrib1 knockdown in organotypic cultures disrupts β-catenin and ZO-2 localization. Morpholino (MO) knockdown of Scrib1 ((C),(D), (G), (H), (K), (L), (O) and (P)) in organotypic cultures compared with control MO ((A), (B), (E), (F), (I), (J), (M) and (N)), followed by immunostaining for junctional proteins revealed selective disruption to key junctional proteins,. β-catenin ((A)–(D) red) and ZO-2 ((M)–(P) red) sub-cellular localisation was significantly disrupted upon Scrib1 knockdown ((C), (D) and (O), (P) compared with (A), (B) and (M), (N)). E-cadherin ((E)–(H) red) did not appear to be visibly altered (compare (G) and (H) with control MO (E) and (F)). ZO-1 ((I)–(L) red) localisation in Scrib1 MO cultures ((K) and (L)) appeared mildly disrupted compared with control MO ((I) and (J)). DAPI staining highlights nuclei ((B), (D), (F), (H), (J), (L), (N) and (P) blue). Scale bars; A–P 5 μM plus 6_x_ zoom.
Fig. 9
Model depicting the potential hierarchy between A/B polarity, planar polarity and branching morphogenesis in the lung. The relationships of Scrib1, Celsr1 and Vangl2 to these processes are indicated (based on the results described here and in Yates et al., 2010).
Similar articles
- Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium.
Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, Auer M. Ewald AJ, et al. J Cell Sci. 2012 Jun 1;125(Pt 11):2638-54. doi: 10.1242/jcs.096875. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344263 Free PMC article. - Scribble and Discs-large direct initial assembly and positioning of adherens junctions during the establishment of apical-basal polarity.
Bonello TT, Choi W, Peifer M. Bonello TT, et al. Development. 2019 Nov 21;146(22):dev180976. doi: 10.1242/dev.180976. Development. 2019. PMID: 31628110 Free PMC article. - A βPIX-PAK2 complex confers protection against Scrib-dependent and cadherin-mediated apoptosis.
Frank SR, Bell JH, Frödin M, Hansen SH. Frank SR, et al. Curr Biol. 2012 Oct 9;22(19):1747-54. doi: 10.1016/j.cub.2012.07.011. Epub 2012 Aug 2. Curr Biol. 2012. PMID: 22863318 Free PMC article. - Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling.
Baum B, Georgiou M. Baum B, et al. J Cell Biol. 2011 Mar 21;192(6):907-17. doi: 10.1083/jcb.201009141. J Cell Biol. 2011. PMID: 21422226 Free PMC article. Review. - The interdependence of the Rho GTPases and apicobasal cell polarity.
Mack NA, Georgiou M. Mack NA, et al. Small GTPases. 2014;5(2):10. doi: 10.4161/21541248.2014.973768. Small GTPases. 2014. PMID: 25469537 Free PMC article. Review.
Cited by
- The Planar Polarity Component VANGL2 Is a Key Regulator of Mechanosignaling.
Cheong SS, Akram KM, Matellan C, Kim SY, Gaboriau DCA, Hind M, Del Río Hernández AE, Griffiths M, Dean CH. Cheong SS, et al. Front Cell Dev Biol. 2020 Oct 29;8:577201. doi: 10.3389/fcell.2020.577201. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195213 Free PMC article. - Polarity scaffolds signaling in epithelial cell permeability.
O'Leary LF, Tomko AM, Dupré DJ. O'Leary LF, et al. Inflamm Res. 2021 May;70(5):525-538. doi: 10.1007/s00011-021-01454-1. Epub 2021 Mar 15. Inflamm Res. 2021. PMID: 33721031 Review. - Scrib is required for epithelial cell identity and prevents epithelial to mesenchymal transition in the mouse.
Yamben IF, Rachel RA, Shatadal S, Copeland NG, Jenkins NA, Warming S, Griep AE. Yamben IF, et al. Dev Biol. 2013 Dec 1;384(1):41-52. doi: 10.1016/j.ydbio.2013.09.027. Epub 2013 Oct 1. Dev Biol. 2013. PMID: 24095903 Free PMC article. - Canonical Wnt signaling activity in early stages of chick lung development.
Moura RS, Carvalho-Correia E, daMota P, Correia-Pinto J. Moura RS, et al. PLoS One. 2014 Dec 2;9(12):e112388. doi: 10.1371/journal.pone.0112388. eCollection 2014. PLoS One. 2014. PMID: 25460002 Free PMC article. - Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap.
Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Szymaniak AD, et al. Dev Cell. 2015 Aug 10;34(3):283-96. doi: 10.1016/j.devcel.2015.06.020. Epub 2015 Jul 30. Dev Cell. 2015. PMID: 26235047 Free PMC article.
References
- Affolter M., Zeller R. From cells to organs: new insight into organ morphogenesis. Curr. Opin. Genetics Dev. 2009;19:421–423. - PubMed
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. - PubMed
- Bilder D., Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HL089431/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL089431/HL/NHLBI NIH HHS/United States
- MC_U142684167/MRC_/Medical Research Council/United Kingdom
- G120/861/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous