Soluble aβ promotes wild-type tau pathology in vivo - PubMed (original) (raw)
Soluble aβ promotes wild-type tau pathology in vivo
Meredith A Chabrier et al. J Neurosci. 2012.
Abstract
Growing evidence suggests that soluble Aβ species can drive Alzheimer disease (AD) pathogenesis by inducing a cascade of events including tau hyperphosphorylation, proteasome impairment, and synaptic dysfunction. However, these studies have relied largely on in vitro approaches to examine the role of soluble Aβ in AD. In particular, it remains unknown whether soluble Aβ oligomers can facilitate the development of human wild-type tau pathology in vivo. To address this question, we developed a novel transgenic model that expresses low levels of APP with the Arctic familial AD mutation to enhance soluble Aβ oligomer formation in conjunction with wild-type human tau. Using a genetic approach, we show that reduction of β-site APP cleaving enzyme (BACE) in these ArcTau mice decreases soluble Aβ oligomers, rescues cognition, and, more importantly, reduces tau accumulation and phosphorylation. Notably, BACE reduction decreases the postsynaptic mislocalization of tau in ArcTau mice and reduces the association between NMDA receptors and PSD-95. These studies provide critical in vivo evidence for a strong mechanistic link between soluble Aβ, wild-type tau, and synaptic pathology.
Figures
Figure 1.
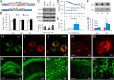
ArcTau mice develop age-related Alzheimer-like pathology and cognitive decline. A, Schematic depiction of human APP and tau transgenes co-microinjected to produce ArcTau transgenic mice. B, ArcTau mice do not display motor deficits at 15 months, as shown by the average time spent on Rotarod compared with WT. C, ArcTau transgenic mice express twofold higher levels of APP (22C11 antibody) and equivalent levels of human and endogenous mouse tau (Tau46 antibody). D, Twelve-month-old ArcTau mice and WT littermate controls were trained in the Morris water maze for 8 d. ArcTau mice performed significantly worse on days 7 and 8 of acquisition. E, ArcTau mice also exhibited poor memory for the platform's former location, as evidenced by significantly longer latencies in a 24 h probe trial. F, ArcTau mice show a significant increase in OC+ fibrillar oligomers at 12 months. G, Representative dot blot of ArcTau half-brain samples at 6, 12, and 18 months. H–J, Histological analysis at 6 months reveals intraneuronal Aβ-like pathology. Full-length APP and Aβ is shown in green (H) and C terminus of APP is stained in red (I); thus, Aβ is detected by green-only puncta in the merge (J). K–M, Wild-type total htau (HT7) is evident throughout the hippocampus (K), specifically CA1 (L), and the cortex (M) at 6 months. N, O, Rare diffuse plaques only begin to appear at 18 months (subiculum shown). P, Q, Hyperphosphorylated (PHF+) somatodendritic wild-type tau is detected in the CA1 at 18 months. N = 8–10 per group; *p < 0.05, **p < 0.01. Error bars represent ± SEM.
Figure 2.
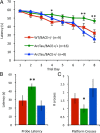
Partial genetic deletion of BACE prevents learning and memory impairments. A, Fifteen-month-old ArcTau, ArcTau/BACE+/−, and WT/BACE+/− were trained in Morris water maze for 8 d. ArcTau/BACE+/− performed similarly to WT/BACE+/− (cognitively normal), while ArcTau/BACE+/+ displayed significant learning deficits on days 4, 7, and 8. B, C, In the 24 h probe trial, ArcTau/BACE+/+ mice took almost twice as long to reach the former platform location and also crossed this area significantly fewer times than ArcTau/BACE+/− and WT/BACE+/− mice. Error bars represent ± SEM; *p < 0.05, **p < 0.01.
Figure 3.
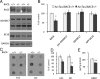
Partial genetic reduction of BACE reduces Arctic Aβ and prefibrillar oligomeric Aβ. A, BACE levels are significantly decreased in ArcTau/BACE+/− compared with ArcTau/BACE+/+ mice, while APP and α-secretase ADAM17 levels are unchanged. B, Quantitation of the Western blots in A by densitometric analysis, shown as percentage of control. C, D, Dot blots from ArcTau animals with BACE knockdown show less fibrillar oligomers, as detected by OC antibody, while levels of prefibrillar oligomers detected by A11 do not vary. E, Levels of Aβ40 are significantly decreased in ArcTau/BACE+/− mice, measured by standard ELISA methods. Aβ42 is undetectable in this model. N = 4–5 per group. Error bars represent ± SEM; *p < 0.05, **p < 0.01.
Figure 4.
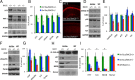
Reduction of soluble Aβ prevents mislocalization and accumulation of wild-type tau. A, B, Total tau levels and tau phosphorylated at pS396/S404 are decreased in ArcTau/BACE+/− mice, whereas other phospho-tau epitopes remain unchanged. C, Somatodendritic wild-type human tau is abundant in ArcTau/ BACE+/+ mice, while barely detectable in the somatodendritic compartments of ArcTau/BACE+/− mice. D, E, Western blots probing for alterations in kinase levels or activity show no differences between ArcTau/BACE+/+ and ArcTau/BACE+/− mice. F, G, There are no statistically significant differences in proteasome activity, as evidenced by levels of CHIP and ubiquitinated proteins, or autophagy (LC3) between ArcTau/BACE+/+ and ArcTau/BACE+/− mice, although there appears to be a buildup of autophagosomes in both ArcTau genotypes compared with wild-type. H, I, Interestingly, coimmunoprecipitation reveals dramatic decreases in the association between PSD-95 and tau, Fyn kinase, and the NR2B subunit of the NMDA receptor in ArcTau/BACE+/− versus ArcTau/BACE+/+ mice. N = 4–5 per group; *p < 0.05 compared with ArcTau/BACE+/+ mice. Error bars represent ± SEM.
Similar articles
- Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport.
Sherman MA, LaCroix M, Amar F, Larson ME, Forster C, Aguzzi A, Bennett DA, Ramsden M, Lesné SE. Sherman MA, et al. J Neurosci. 2016 Sep 14;36(37):9647-58. doi: 10.1523/JNEUROSCI.1899-16.2016. J Neurosci. 2016. PMID: 27629715 Free PMC article. - Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway.
Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M, Ikeuchi T. Tokutake T, et al. J Biol Chem. 2012 Oct 12;287(42):35222-35233. doi: 10.1074/jbc.M112.348300. Epub 2012 Aug 21. J Biol Chem. 2012. PMID: 22910909 Free PMC article. - Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein.
Gomes LA, Hipp SA, Rijal Upadhaya A, Balakrishnan K, Ospitalieri S, Koper MJ, Largo-Barrientos P, Uytterhoeven V, Reichwald J, Rabe S, Vandenberghe R, von Arnim CAF, Tousseyn T, Feederle R, Giudici C, Willem M, Staufenbiel M, Thal DR. Gomes LA, et al. Acta Neuropathol. 2019 Dec;138(6):913-941. doi: 10.1007/s00401-019-02053-5. Epub 2019 Aug 14. Acta Neuropathol. 2019. PMID: 31414210 - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Key Peptides and Proteins in Alzheimer's Disease.
Penke B, Bogár F, Paragi G, Gera J, Fülöp L. Penke B, et al. Curr Protein Pept Sci. 2019;20(6):577-599. doi: 10.2174/1389203720666190103123434. Curr Protein Pept Sci. 2019. PMID: 30605056 Review.
Cited by
- Association of Cortical β-Amyloid Protein in the Absence of Insoluble Deposits With Alzheimer Disease.
Yu L, Petyuk VA, Tasaki S, Boyle PA, Gaiteri C, Schneider JA, De Jager PL, Bennett DA. Yu L, et al. JAMA Neurol. 2019 Jul 1;76(7):818-826. doi: 10.1001/jamaneurol.2019.0834. JAMA Neurol. 2019. PMID: 31009033 Free PMC article. - The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death.
Söllvander S, Nikitidou E, Gallasch L, Zyśk M, Söderberg L, Sehlin D, Lannfelt L, Erlandsson A. Söllvander S, et al. J Neuroinflammation. 2018 Mar 28;15(1):98. doi: 10.1186/s12974-018-1134-4. J Neuroinflammation. 2018. PMID: 29592816 Free PMC article. - Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer's disease immunotherapeutics.
Goure WF, Krafft GA, Jerecic J, Hefti F. Goure WF, et al. Alzheimers Res Ther. 2014 Jul 9;6(4):42. doi: 10.1186/alzrt272. eCollection 2014. Alzheimers Res Ther. 2014. PMID: 25045405 Free PMC article. Review. - Tau Oligomers: The Toxic Player at Synapses in Alzheimer's Disease.
Guerrero-Muñoz MJ, Gerson J, Castillo-Carranza DL. Guerrero-Muñoz MJ, et al. Front Cell Neurosci. 2015 Dec 2;9:464. doi: 10.3389/fncel.2015.00464. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26696824 Free PMC article. Review. - Role of Neurocellular Endoplasmic Reticulum Stress Response in Alzheimer's Disease and Related Dementias Risk.
Aceves M, Granados J, Leandro AC, Peralta J, Glahn DC, Williams-Blangero S, Curran JE, Blangero J, Kumar S. Aceves M, et al. Genes (Basel). 2024 Apr 28;15(5):569. doi: 10.3390/genes15050569. Genes (Basel). 2024. PMID: 38790197 Free PMC article.
References
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
- Cheng IH, Palop JJ, Esposito LA, Bien-Ly N, Yan F, Mucke L. Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med. 2004;10:1190–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 AG039968/AG/NIA NIH HHS/United States
- AG029378/AG/NIA NIH HHS/United States
- P01 AG000538/AG/NIA NIH HHS/United States
- PPG AG00538/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- K01 AG029378/AG/NIA NIH HHS/United States
- R01 AG027544/AG/NIA NIH HHS/United States
- AG027544/AG/NIA NIH HHS/United States
- F31AG039968/AG/NIA NIH HHS/United States
- AG16573/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases