Hypoxia modulates A431 cellular pathways association to tumor radioresistance and enhanced migration revealed by comprehensive proteomic and functional studies - PubMed (original) (raw)
Hypoxia modulates A431 cellular pathways association to tumor radioresistance and enhanced migration revealed by comprehensive proteomic and functional studies
Yan Ren et al. Mol Cell Proteomics. 2013 Feb.
Abstract
Tumor hypoxia induces cancer cell angiogenesis, invasiveness, treatment resistance, and contributes to poor clinical outcome. However, the molecular mechanism by which tumor hypoxia exerts a coordinated effect on different molecular pathways to enhance tumor growth and survival and lead to poor clinical outcome is not fully understood. In this study, we attempt to elucidate the global protein expression and functional changes in A431 epithelial carcinoma cells induced by hypoxia and reoxygenation using iTRAQ quantitative proteomics and biochemical functional assays. Quantitative proteomics results showed that 4316 proteins were quantified with FDR<1%, in which over 1200 proteins were modulated >1.2 fold, and DNA repair, glycolysis, integrin, glycoprotein turnover, and STAT1 pathways were perturbed by hypoxia and reoxygenation-induced oxidative stress. For the first time, hypoxia was shown to up-regulate the nonhomologous end-joining pathway, which plays a central role in DNA repair of irradiated cells, thereby potentially contributing to the radioresistance of hypoxic A431 cells. The up-regulation of Ku70/Ku80 dimer, a key molecular complex in the nonhomologous end-joining pathway, was confirmed by Western blot and liquid chromatography/tandem mass spectrometry-MRM methods. Functional studies confirmed that up-regulation of glycolysis, integrin, glycoprotein synthesis, and down-regulation of STAT1 pathways during hypoxia enhanced metastastic activity of A431 cells. Migration of A431 cells was dramatically repressed by glycolysis inhibitor (2-Deoxy-d-glucose), glycoprotein synthesis inhibitor (1-Deoxynojirimycin Hydrochloride), and STAT1α overexpression that enhanced the integrin-mediated cell adhesion. These results revealed that hypoxia induced several biological processes involved in tumor migration and radioresistance and provided potential new targets for tumor therapy.
Figures
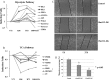
Fig. 1.
Hypoxia activates NHEJ pathway proteins mainly responsible for DNA repair under irradiation. A, Change patterns of NHEJ pathway proteins because of hypoxia or reoxygenation from iTRAQ data; B, The increase of Ku70, Ku80 and their heterodimer induced by hypoxia was confirmed by Western blot; In these WB experiments, the cell proteins were extracted by the solution of 1% Triton X-100 in 20 m
m
Tris-HCl (pH 7.4), 150 m
m
NaCl. Ku70 and Ku80 monomers were separated by SDS-PAGE and detected by individual antibody (the upper three panels). For the dimer quantification, the same proteins with those loaded on SDS-PAGE gel were separated in a native gel and the dimer was detected by both antibodies (the lower two panels). C, The increase of three components of DNA-PK heterotrimer, Ku70, Ku80, and PRKDC1 was further proved by MRM. The retention time for PRKDC1 had some shift between Nx and Hx samples for some unknown reasons. The other transitions also occurred with the same shift between the two samples. Hx, Hypoxic sample; Nx, Normoxic sample.
Fig. 2.
Hypoxia activates glycolysis and inhibits Kreb cycles by modulating the enzymes involved in the pathways. A and B, Change pattern of enzymes involved in glycolysis and pyruvate metabolism induced by hypoxia or reoxygenation; C, Images of scratch assay of A431 treated with glycolysis inhibitor, 2-DG, for 20 h, Magnification, ×100; D, The effect of 2-DG on inhibition of cell migration at 10 m
m
and 15 m
m
concentration.
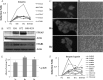
Fig. 3.
Hypoxia increases the expression level of integrin subunits and their recruitment to plasma membrane and even induces the expression of their partners. A, Change patterns of integrin subunits because of hypoxia or reoxygenation from iTRAQ data; B, The higher expression level of ITGA5 and ITGB1 under hypoxia was confirmed by Western blot. C, Cell immunostaining of ITGA5 clearly indicated that the adhesion molecule was prone to localize at the cell membrane under hypoxia. Magnification, ×630; D and E, Images and quantification of adhesion assay of A431 to FN under Nx, Hx, and Reox condition. Magnification, ×100; F, Change patterns of integrin ligands because of hypoxia or reoxygenation from iTRAQ data.
Fig. 4.
Hypoxia speeds up the turnover of glycoproteins in A431. A and B, Change patterns of enzymes involved in glycan biosynthesis and glycoprotein degradation during hypoxia or reoxygenation; Glycoprotein degradation has two steps: proteolysis and glycan degradation. The two sets of enzymes have similar change pattern responsible to hypoxia or reoxygenation. C, Images of scratch assay of A431 treated with 100 μ
m
1-DJ for 48 h first to suppress glycoprotein synthesis, Magnification, ×100; D, The inhibition of glycoprotein synthesis after 1-DJ treatment was verified in lectin binding assay by WB; E, The effect of 1-DJ in inhibition of cell migration at 100 μ
m
concentration.
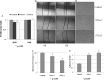
Fig. 5.
Confirmation of STAT1 pathway suppressed by hypoxia and validation of stable cell lines with STAT1α overexpression in A431; A, Change patterns of STAT1 and its downstream proteins because of hypoxia or reoxygenation; B, The down-regulation of STAT1 and pSTAT1α (S727) was confirmed by WB. C, The decrease of functional STAT1 represses the transcription of its downstream genes. D, The two positive stable cell lines with STAT1α overexpression were confirmed by WB using anti-FLAG, STAT1 and pSTAT1α (S727) antibodies. E, The activation of downstream genes of STAT1 in the stable cell lines indicated that the cell lines indeed have more active STAT1α.
Fig. 6.
Overexpression of STAT1α suppresses the migration and enhances the adhesion of A431. A, MTT assay under Nx and Hx; B and C, Images and quantification of scratch assay of control and STAT1α overexpression A431 cells, Magnification, ×100; D and E, Images and quantification of adhesion assay of control and STAT1 overexpression A431 cells
Similar articles
- RON Nuclear Translocation under Hypoxia Potentiates Chemoresistance to DNA Double-Strand Break-Inducing Anticancer Drugs.
Chang HY, Chang TC, Huang WY, Lee CT, Yen CJ, Tsai YS, Tzai TS, Chen SH, Chow NH. Chang HY, et al. Mol Cancer Ther. 2016 Feb;15(2):276-86. doi: 10.1158/1535-7163.MCT-15-0311. Epub 2016 Jan 15. Mol Cancer Ther. 2016. PMID: 26772202 - Adenovirus-mediated expression of a dominant negative Ku70 fragment radiosensitizes human tumor cells under aerobic and hypoxic conditions.
He F, Li L, Kim D, Wen B, Deng X, Gutin PH, Ling CC, Li GC. He F, et al. Cancer Res. 2007 Jan 15;67(2):634-42. doi: 10.1158/0008-5472.CAN-06-1860. Cancer Res. 2007. PMID: 17234773 - An SCF complex containing Fbxl12 mediates DNA damage-induced Ku80 ubiquitylation.
Postow L, Funabiki H. Postow L, et al. Cell Cycle. 2013 Feb 15;12(4):587-95. doi: 10.4161/cc.23408. Epub 2013 Jan 16. Cell Cycle. 2013. PMID: 23324393 Free PMC article. - The Ku heterodimer: function in DNA repair and beyond.
Fell VL, Schild-Poulter C. Fell VL, et al. Mutat Res Rev Mutat Res. 2015 Jan-Mar;763:15-29. doi: 10.1016/j.mrrev.2014.06.002. Epub 2014 Jul 4. Mutat Res Rev Mutat Res. 2015. PMID: 25795113 Review. - Destroying the ring: Freeing DNA from Ku with ubiquitin.
Postow L. Postow L. FEBS Lett. 2011 Sep 16;585(18):2876-82. doi: 10.1016/j.febslet.2011.05.046. Epub 2011 Jun 1. FEBS Lett. 2011. PMID: 21640108 Free PMC article. Review.
Cited by
- Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance.
Krymov SK, Scherbakov AM, Dezhenkova LG, Salnikova DI, Solov'eva SE, Sorokin DV, Vullo D, De Luca V, Capasso C, Supuran CT, Shchekotikhin AE. Krymov SK, et al. Pharmaceuticals (Basel). 2022 Nov 23;15(12):1453. doi: 10.3390/ph15121453. Pharmaceuticals (Basel). 2022. PMID: 36558903 Free PMC article. - Proteomics analyses for the global proteins in the brain tissues of different human prion diseases.
Shi Q, Chen LN, Zhang BY, Xiao K, Zhou W, Chen C, Zhang XM, Tian C, Gao C, Wang J, Han J, Dong XP. Shi Q, et al. Mol Cell Proteomics. 2015 Apr;14(4):854-69. doi: 10.1074/mcp.M114.038018. Epub 2015 Jan 23. Mol Cell Proteomics. 2015. PMID: 25616867 Free PMC article. - 18F-Fluoromisonidazole in tumor hypoxia imaging.
Xu Z, Li XF, Zou H, Sun X, Shen B. Xu Z, et al. Oncotarget. 2017 Oct 7;8(55):94969-94979. doi: 10.18632/oncotarget.21662. eCollection 2017 Nov 7. Oncotarget. 2017. PMID: 29212283 Free PMC article. Review. - Application of Advanced Mass Spectrometry-Based Proteomics to Study Hypoxia Driven Cancer Progression.
Vinaiphat A, Low JK, Yeoh KW, Chng WJ, Sze SK. Vinaiphat A, et al. Front Oncol. 2021 Feb 23;11:559822. doi: 10.3389/fonc.2021.559822. eCollection 2021. Front Oncol. 2021. PMID: 33708620 Free PMC article. Review. - Inside the hypoxic tumour: reprogramming of the DDR and radioresistance.
Begg K, Tavassoli M. Begg K, et al. Cell Death Discov. 2020 Aug 18;6:77. doi: 10.1038/s41420-020-00311-0. eCollection 2020. Cell Death Discov. 2020. PMID: 32864165 Free PMC article. Review.
References
- Brown J. M., Giaccia A. J. (1998) The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58, 1408–1416 - PubMed
- Harris A. L. (2002) Hypoxia–a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 - PubMed
- Wilson W. R., Hay M. P. (2011) Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 - PubMed
- Gray L. H., Conger A. D., Ebert M., Hornsey S., Scott O. C. (1953) The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 26, 638–648 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous