An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer - PubMed (original) (raw)
An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer
Christoph Garbers et al. J Biol Chem. 2013.
Abstract
IL-27 consists of the cytokine subunit p28 and the non-signaling α-receptor EBI3. p28 was shown to additionally act via the non-signaling membrane-bound IL-6 α-receptor (IL-6R) as an agonistic cytokine but also as a gp130 β-receptor antagonist, leading to inhibition of IL-6 signaling. Here, we developed a strategy for bacterial expression, purification, and refolding of murine p28. We show that p28 did not interfere with IL-6- or IL-27-induced signaling, indicating that p28 has no antagonistic properties. Moreover, we demonstrate that murine p28 acts as an agonistic cytokine via the murine and human IL-6R, indicating that p28 exhibits no species specificity. p28 was able to induce p28-trans-signaling via the soluble IL-6R (sIL-6R), a characteristic property that was initially described for trans-signaling of IL-6 via the sIL-6R. Of notice, p28/sIL-6R trans-signaling was inhibited by the IL-6 trans-signaling antagonist, soluble gp130. At higher concentrations, p28 but not IL-6 was able to induce signaling even in the absence of IL-6R or EBI3. Although IL-27 signals via a heterodimer of the β-receptor chains gp130 and Wsx-1, p28/IL-6R specifically recruits two gp130 β-receptor chains for signal transduction. The binding of p28 to a gp130/Wsx-1 heterodimer or a gp130 homodimer is highly selective and controlled by a novel molecular switch induced by EBI3 or IL-6R, respectively.
Figures
FIGURE 1.
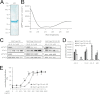
Bacterially produced p28 is an agonistic cytokine. A, SDS-PAGE and Coomassie staining of purified monomeric p28. Two bands containing p28 of slightly differing size were visible. The weaker and lower band contains most probably degraded p28. B, CD spectrum of refolded p28. The shape of the spectrum indicated an α-helical conformation. C, equal numbers of Ba/F3-gp130, Ba/F3-gp130-hIL-6R, and Ba/F3-gp130-mIL-6R cells were stimulated for 15 min with 10 ng/ml Hyper-IL-6 (HIL-6), 10 ng/ml human IL-6, or 200 ng/ml murine p28 or were left unstimulated. Phosphorylation of STAT1 and STAT3 was assessed by Western blotting. Determination of STAT1 and STAT3 protein levels served as loading controls. D, equal numbers of Ba/F3-gp130, Ba/F3-gp130-hIL-6R, and Ba/F3-gp130-mIL-6R cells were cultured for 2 days with 10 ng/ml Hyper-IL-6, 10 ng/ml human IL-6 (hIL-6), 10 ng/ml murine IL-6 (mIL-6) or 200 ng/ml murine p28 (p28) or were left unstimulated. E, equal numbers of Ba/F3-gp130-hIL-6R and Ba/F3-gp130-mIL-6R cells were cultured for 2 days with different concentrations of murine p28 (0–4,000 ng/ml). Cellular proliferation in D and E was quantified as indicated under “Experimental Procedures.”
FIGURE 2.
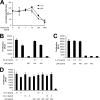
p28 is not an antagonistic cytokine and is inhibited by tocilizumab. A, equal numbers of Ba/F3-gp130-hIL-6R cells were cultured for 2 days with 10 ng/ml human IL-6 or 200 ng/ml murine p28 and with different concentrations of the anti-human-IL-6R antibody tocilizumab (0–1000 ng/ml), respectively. B, equal numbers of Ba-F3-gp130 cells were cultured for 2 days with 1 or 10 ng/ml Hyper-IL-6 (HIL-6) in the presence or absence of 200 or 400 ng/ml murine p28. C, equal numbers of Ba/F3-gp130 cells were cultured for 2 days with 0.5 ng/ml IL-27 in the presence or absence of 200 ng/ml murine p28. D, equal numbers of Ba/F3-gp130-mIL-6R cells were cultured for 2 days with 5 or 10 ng/ml human IL-6 (hIL-6) or 5 or 10 ng/ml murine IL-6 (mIL-6) in the presence or absence of 200 ng/ml murine p28. Cellular proliferation in A–D was quantified as indicated under “Experimental Procedures.”
FIGURE 3.
p28 trans-signaling via the soluble IL-6R is inhibited by sgp130Fc. A, equal numbers of Ba/F3-gp130 cells were stimulated with p28/sIL-6R-FP- or IL-27-conditioned media from transiently transfected COS7 cells for 15 min or left untreated. Conditioned medium was supplemented with sgp130Fc 30 min prior to stimulation where indicated. Cell lysates were analyzed via Western blotting with antibodies against pSTAT3 and against STAT3 as a loading control. B, equal numbers of Ba/F3-gp130 cells were cultured for 2 days with p28/sIL-6R-FP-conditioned medium in the presence or absence of increasing concentrations of sgp130Fc (0.01–1000 ng/ml). As a control, supernatant from enhanced GFP-expressing (EGFP) COS7 cells was used. Cellular proliferation was quantified as indicated under “Experimental Procedures.”
FIGURE 4.
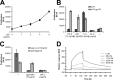
p28 can signal via gp130 without the α-receptors EBI3 and IL-6R. A, equal numbers of Ba/F3-gp130 cells were incubated with increasing concentrations of p28 (0–8000 ng/ml) for 2 days. B, equal numbers of Ba/F3 or Ba/F3-gp130 cells were cultured for 2 days with cytokines at the indicated concentrations or left unstimulated. hIL-6, human IL-6; HIL-6, Hyper-IL-6. C, equal numbers of Ba/F3-gp130 cells were cultured for 2 days with either 10 ng/ml Hyper-IL-6 or 4 μg/ml p28. sgp130Fc (10 μg/ml) and BR-3 (1 μg/ml) were tested for their ability to inhibit p28-induced Ba/F3-gp130 proliferation. In A–C, cellular proliferation was quantified as indicated under “Experimental Procedures.” D, surface plasmon resonance measurements of binding of p28 to sgp130Fc as indicated under “Experimental Procedures.”
FIGURE 5.
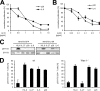
p28/IL-6R-induced signaling is mediated by a homodimer of the β-receptor chain gp130. A, Ba/F3-gp130-mIL-6R cells were cultured for 2 days with 10 ng/ml human IL-6 (hIL-6) or 200 ng/ml murine p28 and increasing concentrations of the gp130 antibody BT-2 (0–10 μg/ml). B, Ba/F3-gp130-mIL-6R cells were cultured for 2 days with 10 ng/ml human IL-6 or 200 ng/ml murine p28 and increasing concentrations of the gp130 antibody BR-3 (0–1 μg/ml). In A and B, cellular proliferation was quantified as indicated under “Experimental Procedures.” C, NIH3T3 cells were transiently transfected with plasmids coding for the human IL-6R or enhanced GFP (EGFP). Cells were stimulated with Hyper-IL-6 (HIL-6, 10 ng/ml), IL-27 (10 ng/ml), p28 (200 ng/ml), or IL-6 (50 ng/ml) for 15 min or were left untreated. Phosphorylation of STAT3 was assessed by Western blotting. Determination of STAT3 protein levels served as loading control. D, purified CD4+ T cells from wild-type and from Wsx-1-deficient mice were stimulated with Hyper-IL-6 (10 ng/ml), IL-27 (10 ng/ml), IL-6 (10 ng/ml), or p28 (200 ng/ml) for 15 min or were left untreated. STAT3 phosphorylation was determined by flow cytometry and normalized.
Similar articles
- The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling.
Crabé S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I, Ferlin W, Elson G, Jeannin P, Gauchat JF. Crabé S, et al. J Immunol. 2009 Dec 15;183(12):7692-702. doi: 10.4049/jimmunol.0901464. J Immunol. 2009. PMID: 19933857 - Structure-guided optimization of the interleukin-6 trans-signaling antagonist sgp130.
Tenhumberg S, Waetzig GH, Chalaris A, Rabe B, Seegert D, Scheller J, Rose-John S, Grötzinger J. Tenhumberg S, et al. J Biol Chem. 2008 Oct 3;283(40):27200-7. doi: 10.1074/jbc.M803694200. Epub 2008 Jul 23. J Biol Chem. 2008. PMID: 18650419 - Epstein-Barr virus-induced gene 3 (EBI3) can mediate IL-6 _trans_-signaling.
Chehboun S, Labrecque-Carbonneau J, Pasquin S, Meliani Y, Meddah B, Ferlin W, Sharma M, Tormo A, Masson JF, Gauchat JF. Chehboun S, et al. J Biol Chem. 2017 Apr 21;292(16):6644-6656. doi: 10.1074/jbc.M116.762021. Epub 2017 Mar 9. J Biol Chem. 2017. PMID: 28280243 Free PMC article. - Interleukin-6 and its receptors: a highly regulated and dynamic system.
Wolf J, Rose-John S, Garbers C. Wolf J, et al. Cytokine. 2014 Nov;70(1):11-20. doi: 10.1016/j.cyto.2014.05.024. Epub 2014 Jun 28. Cytokine. 2014. PMID: 24986424 Review. - IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6.
Rose-John S. Rose-John S. Int J Biol Sci. 2012;8(9):1237-47. doi: 10.7150/ijbs.4989. Epub 2012 Oct 24. Int J Biol Sci. 2012. PMID: 23136552 Free PMC article. Review.
Cited by
- IL-30† (IL-27A): a familiar stranger in immunity, inflammation, and cancer.
Min B, Kim D, Feige MJ. Min B, et al. Exp Mol Med. 2021 May;53(5):823-834. doi: 10.1038/s12276-021-00630-x. Epub 2021 May 28. Exp Mol Med. 2021. PMID: 34045653 Free PMC article. Review. - Interleukin-27 Functional Duality Balances Leishmania Infectivity and Pathogenesis.
Jafarzadeh A, Nemati M, Chauhan P, Patidar A, Sarkar A, Sharifi I, Saha B. Jafarzadeh A, et al. Front Immunol. 2020 Aug 7;11:1573. doi: 10.3389/fimmu.2020.01573. eCollection 2020. Front Immunol. 2020. PMID: 32849534 Free PMC article. Review. - Evaluating the potential of IL-27 as a novel therapeutic agent in HIV-1 infection.
Swaminathan S, Dai L, Lane HC, Imamichi T. Swaminathan S, et al. Cytokine Growth Factor Rev. 2013 Dec;24(6):571-7. doi: 10.1016/j.cytogfr.2013.07.001. Epub 2013 Aug 17. Cytokine Growth Factor Rev. 2013. PMID: 23962745 Free PMC article. Review. - IL-6 as a keystone cytokine in health and disease.
Hunter CA, Jones SA. Hunter CA, et al. Nat Immunol. 2015 May;16(5):448-57. doi: 10.1038/ni.3153. Nat Immunol. 2015. PMID: 25898198 Review. - The molecular basis of chaperone-mediated interleukin 23 assembly control.
Meier S, Bohnacker S, Klose CJ, Lopez A, Choe CA, Schmid PWN, Bloemeke N, Rührnößl F, Haslbeck M, Bieren JE, Sattler M, Huang PS, Feige MJ. Meier S, et al. Nat Commun. 2019 Sep 11;10(1):4121. doi: 10.1038/s41467-019-12006-x. Nat Commun. 2019. PMID: 31511508 Free PMC article.
References
- Garbers C., Hermanns H. M., Schaper F., Müller-Newen G., Grötzinger J., Rose-John S., Scheller J. (2012) Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 23, 85–97 - PubMed
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 - PubMed
- Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J. F., Phillips J. H., McClanahan T. K., de Waal Malefyt R., Kastelein R. A. (2004) WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials