Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy - PubMed (original) (raw)
Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy
Zhiwei Huang et al. PLoS Genet. 2012.
Abstract
Chloroquine (CQ) and other quinoline-containing antimalarials are important drugs with many therapeutic benefits as well as adverse effects. However, the molecular targets underlying most such effects are largely unknown. By taking a novel functional genomics strategy, which employs a unique combination of genome-wide drug-gene synthetic lethality (DGSL), gene-gene synthetic lethality (GGSL), and dosage suppression (DS) screens in the model organism Saccharomyces cerevisiae and is thus termed SL/DS for simplicity, we found that CQ inhibits the thiamine transporters Thi7, Nrt1, and Thi72 in yeast. We first discovered a thi3Δ mutant as hypersensitive to CQ using a genome-wide DGSL analysis. Using genome-wide GGSL and DS screens, we then found that a thi7Δ mutation confers severe growth defect in the thi3Δ mutant and that THI7 overexpression suppresses CQ-hypersensitivity of this mutant. We subsequently showed that CQ inhibits the functions of Thi7 and its homologues Nrt1 and Thi72. In particular, the transporter activity of wild-type Thi7 but not a CQ-resistant mutant (Thi7(T287N)) was completely inhibited by the drug. Similar effects were also observed with other quinoline-containing antimalarials. In addition, CQ completely inhibited a human thiamine transporter (SLC19A3) expressed in yeast and significantly inhibited thiamine uptake in cultured human cell lines. Therefore, inhibition of thiamine uptake is a conserved mechanism of action of CQ. This study also demonstrated SL/DS as a uniquely effective methodology for discovering drug targets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
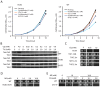
Figure 1. CQ possibly affects multiple biological functions.
A. Representative yeast haploid deletion mutants exhibiting the highest levels of sensitivity to CQ. B. Suppression of CQ-hypersensitivity of the fet3Δ and thi3Δ mutants by exogenously supplied iron [Fe(III)] and thiamine (Thi), respectively. C. Yeast tetrad analysis of the synthetically sick interaction between thi3Δ and thi7Δ. Colonies of the thi3Δ thi7Δ double mutant were all circled. D. Increased CQ-sensitivity of a thi3Δ thi7Δ double mutant as compared to either single mutant. E. Dosage-dependent suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by exogenously supplied thiamine. F. Suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by overexpressing THI3, THI7, and PDC2. G. Suppression of CQ-hypersensitivity of the thi3Δ single mutant by overexpressing THI7 and PDC2.
Figure 2. CQ-treatment mimics the effects of inactivating thiamine transporters.
A. CQ partially suppresses Thi7-dependent toxicity of pyrithiamine. Growth curves of a wild-type (WT) strain and a thi7Δ mutant under the indicated conditions were plotted. B. Thi3-dependent induction of expression of a Thi6-TAP fusion protein by CQ-treatment, a thi7Δ mutation, and thiamine deprivation. The fusion protein was detected with an anti-TAP antibody. Tub2 was used as a loading control. C. Suppression of CQ-hypersensitivity of the thi3Δ thi7Δ double mutant by expressing THI3, THI7, NRT1, or THI72 from a centromeric (CEN) or high copy (2µ) plasmid. D. Excess amount of nicotinamide riboside (NR) inhibits growth of the thi3Δ thi7Δ double mutant. E. CQ inhibits NR-dependent growth of a qns1Δ mutant.
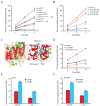
Figure 3. CQ inhibits thiamine transporters from yeast and human.
A. CQ (1.0 mM) completely inhibits thiamine uptake mediated by wild-type Thi7 but not CQ-resistant mutants of indicated genotypes. B. CQ dose-dependent inhibition of the transporter activity of wild-type Thi7. C. A homology model of the closed form of Thi7. The transmembrane (TM) helices are shown in red with the membrane region shown in a peach background. The black dashed rectangle is shown on the right in detail with docked substrates. The lowest energy docking results of thiamine (magenta) and chloroquine (cyan) are shown, with the side chain of T287 depicted in orange. D. CQ (1.0 mM) completely inhibits the human thiamine transporter SLC19A3 expressed in yeast cells. E and F. CQ (0.25 mM) inhibits thiamine uptake in human HeLa (E) and HT1080 (F) cell lines.
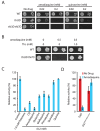
Figure 4. Other antimalarials inhibit thiamine transporters.
A. Hypersensitivity of the thi3Δ and thi3Δ thi7Δ mutants to amodiaquine and quinacrine. B. Suppression of amodiaquine-hypersensitivity of the thi3Δ and thi3Δ thi7Δ mutants by exogenously supplied thiamine. C. Inhibition of thiamine uptake by indicated compounds. D. Amodiaquine (0.25 mM) inhibits thiamine uptake mediated by wild-type Thi7 but not a CQ-resistant Thi7R9G T287N E573G mutant.
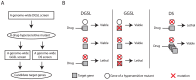
Figure 5. A diagram and concept of discovering drug target using the SL/DS strategy.
A flow chart (A) and concept (B) of drug target identification with a combination of DGSL, GGSL, and DS screens.
Similar articles
- Identification of yeast and human 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAr) transporters.
Ceschin J, Saint-Marc C, Laporte J, Labriet A, Philippe C, Moenner M, Daignan-Fornier B, Pinson B. Ceschin J, et al. J Biol Chem. 2014 Jun 13;289(24):16844-54. doi: 10.1074/jbc.M114.551192. Epub 2014 Apr 28. J Biol Chem. 2014. PMID: 24778186 Free PMC article. - Yeast α-arrestin Art2 is the key regulator of ubiquitylation-dependent endocytosis of plasma membrane vitamin B1 transporters.
Savocco J, Nootens S, Afokpa W, Bausart M, Chen X, Villers J, Renard HF, Prévost M, Wattiez R, Morsomme P. Savocco J, et al. PLoS Biol. 2019 Oct 28;17(10):e3000512. doi: 10.1371/journal.pbio.3000512. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31658248 Free PMC article. - A Redox-Active Fluorescent pH Indicator for Detecting Plasmodium falciparum Strains with Reduced Responsiveness to Quinoline Antimalarial Drugs.
Jida M, Sanchez CP, Urgin K, Ehrhardt K, Mounien S, Geyer A, Elhabiri M, Lanzer M, Davioud-Charvet E. Jida M, et al. ACS Infect Dis. 2017 Feb 10;3(2):119-131. doi: 10.1021/acsinfecdis.5b00141. Epub 2016 Dec 7. ACS Infect Dis. 2017. PMID: 28183182 - Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum.
Sanchez CP, Stein WD, Lanzer M. Sanchez CP, et al. Trends Parasitol. 2007 Jul;23(7):332-9. doi: 10.1016/j.pt.2007.04.013. Epub 2007 May 10. Trends Parasitol. 2007. PMID: 17493873 Review. - Current understanding of the molecular basis of chloroquine-resistance in Plasmodium falciparum.
Jiang H, Joy DA, Furuya T, Su XZ. Jiang H, et al. J Postgrad Med. 2006 Oct-Dec;52(4):271-6. J Postgrad Med. 2006. PMID: 17102545 Review.
Cited by
- Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi.
Peyclit L, Yousfi H, Rolain JM, Bittar F. Peyclit L, et al. Pharmaceuticals (Basel). 2021 May 20;14(5):488. doi: 10.3390/ph14050488. Pharmaceuticals (Basel). 2021. PMID: 34065420 Free PMC article. Review. - Chloroquine and hydroxychloroquine for COVID-19: Perspectives on their failure in repurposing.
Shah RR. Shah RR. J Clin Pharm Ther. 2021 Feb;46(1):17-27. doi: 10.1111/jcpt.13267. Epub 2020 Sep 27. J Clin Pharm Ther. 2021. PMID: 32981089 Free PMC article. Review. - iSeq: A New Double-Barcode Method for Detecting Dynamic Genetic Interactions in Yeast.
Jaffe M, Sherlock G, Levy SF. Jaffe M, et al. G3 (Bethesda). 2017 Jan 5;7(1):143-153. doi: 10.1534/g3.116.034207. G3 (Bethesda). 2017. PMID: 27821633 Free PMC article. - Identification of yeast and human 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAr) transporters.
Ceschin J, Saint-Marc C, Laporte J, Labriet A, Philippe C, Moenner M, Daignan-Fornier B, Pinson B. Ceschin J, et al. J Biol Chem. 2014 Jun 13;289(24):16844-54. doi: 10.1074/jbc.M114.551192. Epub 2014 Apr 28. J Biol Chem. 2014. PMID: 24778186 Free PMC article. - Existing Drugs Considered as Promising in COVID-19 Therapy.
Janik E, Niemcewicz M, Podogrocki M, Saluk-Bijak J, Bijak M. Janik E, et al. Int J Mol Sci. 2021 May 21;22(11):5434. doi: 10.3390/ijms22115434. Int J Mol Sci. 2021. PMID: 34063964 Free PMC article. Review.
References
- Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625: 220–233. - PubMed
- Tehrani R, Ostrowski RA, Hariman R, Jay WM (2008) Ocular toxicity of hydroxychloroquine. Semin Ophthalmol 23: 201–209. - PubMed
- Costedoat-Chalumeau N, Hulot JS, Amoura Z, Delcourt A, Maisonobe T, et al. (2007) Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology 107: 73–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases