Non-synonymous polymorphisms in the FCN1 gene determine ligand-binding ability and serum levels of M-ficolin - PubMed (original) (raw)
Non-synonymous polymorphisms in the FCN1 gene determine ligand-binding ability and serum levels of M-ficolin
Christian Gytz Ammitzbøll et al. PLoS One. 2012.
Abstract
Background: The innate immune system encompasses various recognition molecules able to sense both exogenous and endogenous danger signals arising from pathogens or damaged host cells. One such pattern-recognition molecule is M-ficolin, which is capable of activating the complement system through the lectin pathway. The lectin pathway is multifaceted with activities spanning from complement activation to coagulation, autoimmunity, ischemia-reperfusion injury and embryogenesis. Our aim was to explore associations between SNPs in FCN1, encoding M-ficolin and corresponding protein concentrations, and the impact of non-synonymous SNPs on protein function.
Principal findings: We genotyped 26 polymorphisms in the FCN1 gene and found 8 of these to be associated with M-ficolin levels in a cohort of 346 blood donors. Four of those polymorphisms were located in the promoter region and exon 1 and were in high linkage disequilibrium (r(2)≥0.91). The most significant of those were the AA genotype of -144C>A (rs10117466), which was associated with an increase in M-ficolin concentration of 26% compared to the CC genotype. We created recombinant proteins corresponding to the five non-synonymous mutations encountered and found that the Ser268Pro (rs150625869) mutation lead to loss of M-ficolin production. This was backed up by clinical observations, indicating that an individual homozygote of Ser268Pro would be completely M-ficolin deficient. Furthermore, the Ala218Thr (rs148649884) and Asn289Ser (rs138055828) were both associated with low M-ficolin levels, and the mutations crippled the ligand-binding capability of the recombinant M-ficolin, as indicated by the low binding to Group B Streptococcus.
Significance: Overall, our study interlinks the genotype and phenotype relationship concerning polymorphisms in FCN1 and corresponding concentrations and biological functions of M-ficolin. The elucidations of these associations provide information for future genetic studies in the lectin pathway and complement system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
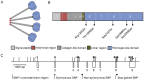
Figure 1. The structural and domain organization of M-ficolin and the organization of the exons in FCN1. A
M-ficolin oligomer consisting of 4 subunits each made of 3 identical polypeptides. B Structure of the M-ficolin polypeptide. White numbers indicate exon and dotted line indicate exons boundaries. The 5 non-synonymous SNPs encountered in the cohort are marked. Amino acid numbers include the signal peptide of 29 residues. C Representation of the promoter, exon and intron region of FCN1 drawn to scale. Exons are marked as boxes below the line and SNPs as lines above. All 26 SNPs genotyped in the cohort are marked.
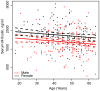
Figure 2. Association between age and serum concentration of M-ficolin split by gender.
Full-drawn lines represents the estimated linear association for males (red) and females (black). Dotted lines represent 95% pointwise confidence intervals.
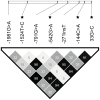
Figure 3. Correlation between the SNPs in the promoter region and exon 1 (R2 values).
R2 is given as percent. Stars indicate SNPs that are significantly associated with M-ficolin concentration.
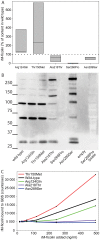
Figure 4. Characterization of five recombinant M-ficolin proteins. A
The M-ficolin concentration measured in the supernatant of HEK293F cells transfected with plasmid encoding variants of M-ficolin. The wild type used as reference and the dotted line represents this value (100%). Boxes indicate range of data including median value. B Western blotting of supernatant from the wild type and the five variants of M-ficolin. For Ser268Pro also a lysate of the cells were used. The mutation for each variant is given beneath the lane. C Binding of recombinant M-ficolin to Streptococcus agalactiae serotype VI (GBS) The counts on the y-axis were obtained following incubation in GBS coated wells with recombinant M-ficolin and anti-M-ficolin antibody. Results displayed in A are from three while B and C are from two replicated experiments.
Similar articles
- Ficolin-1 and ficolin-3 polymorphisms and susceptibility to rheumatoid arthritis.
Pieczarka C, Andrade FA, Catarino SJ, Lidani KCF, Bavia L, Tizzot R, Skare T, de Messias-Reason IJ. Pieczarka C, et al. Autoimmunity. 2020 Nov;53(7):400-407. doi: 10.1080/08916934.2020.1809654. Epub 2020 Aug 21. Autoimmunity. 2020. PMID: 32820945 - Susceptibility to leprosy is associated with M-ficolin polymorphisms.
Boldt AB, Sanchez MI, Stahlke ER, Steffensen R, Thiel S, Jensenius JC, Prevedello FC, Mira MT, Kun JF, Messias-Reason IJ. Boldt AB, et al. J Clin Immunol. 2013 Jan;33(1):210-9. doi: 10.1007/s10875-012-9770-4. Epub 2012 Sep 1. J Clin Immunol. 2013. PMID: 22941510 - Variation in FCN1 affects biosynthesis of ficolin-1 and is associated with outcome of systemic inflammation.
Munthe-Fog L, Hummelshoj T, Honoré C, Moller ME, Skjoedt MO, Palsgaard I, Borregaard N, Madsen HO, Garred P. Munthe-Fog L, et al. Genes Immun. 2012 Oct;13(7):515-22. doi: 10.1038/gene.2012.27. Epub 2012 Jun 7. Genes Immun. 2012. PMID: 22673311 - The genetics of ficolins.
Garred P, Honoré C, Ma YJ, Rørvig S, Cowland J, Borregaard N, Hummelshøj T. Garred P, et al. J Innate Immun. 2010;2(1):3-16. doi: 10.1159/000242419. Epub 2009 Sep 24. J Innate Immun. 2010. PMID: 20375618 Review. - MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement.
Garred P, Honoré C, Ma YJ, Munthe-Fog L, Hummelshøj T. Garred P, et al. Mol Immunol. 2009 Sep;46(14):2737-44. doi: 10.1016/j.molimm.2009.05.005. Epub 2009 Jun 7. Mol Immunol. 2009. PMID: 19501910 Review.
Cited by
- Sickening or Healing the Heart? The Association of Ficolin-1 and Rheumatic Fever.
Catarino SJ, Andrade FA, Boldt ABW, Guilherme L, Messias-Reason IJ. Catarino SJ, et al. Front Immunol. 2018 Dec 18;9:3009. doi: 10.3389/fimmu.2018.03009. eCollection 2018. Front Immunol. 2018. PMID: 30619357 Free PMC article. - CRP genotype and haplotype associations with serum C-reactive protein level and DAS28 in untreated early rheumatoid arthritis patients.
Ammitzbøll CG, Steffensen R, Bøgsted M, Hørslev-Petersen K, Hetland ML, Junker P, Johansen JS, Pødenphant J, Østergaard M, Ellingsen T, Stengaard-Pedersen K. Ammitzbøll CG, et al. Arthritis Res Ther. 2014 Oct 31;16(5):475. doi: 10.1186/s13075-014-0475-3. Arthritis Res Ther. 2014. PMID: 25359432 Free PMC article. - The Influence of the Lectin Pathway of Complement Activation on Infections of the Respiratory System.
Świerzko AS, Cedzyński M. Świerzko AS, et al. Front Immunol. 2020 Oct 21;11:585243. doi: 10.3389/fimmu.2020.585243. eCollection 2020. Front Immunol. 2020. PMID: 33193407 Free PMC article. Review. - The clinical value of ficolin-3 gene polymorphism in rheumatic heart disease. An Egyptian adolescents study.
Gomaa MH, Khidr EG, Elshafei A, Hamza HS, Fattouh AM, El-Husseiny AA, Aglan A, Eldeib MG. Gomaa MH, et al. BMC Res Notes. 2021 Jan 26;14(1):36. doi: 10.1186/s13104-021-05450-w. BMC Res Notes. 2021. PMID: 33499929 Free PMC article. - Fibrinogen-Related Proteins in Tissue Repair: How a Unique Domain with a Common Structure Controls Diverse Aspects of Wound Healing.
Zuliani-Alvarez L, Midwood KS. Zuliani-Alvarez L, et al. Adv Wound Care (New Rochelle). 2015 May 1;4(5):273-285. doi: 10.1089/wound.2014.0599. Adv Wound Care (New Rochelle). 2015. PMID: 26005593 Free PMC article. Review.
References
- Degn SE, Jensenius JC, Bjerre M (2010) The lectin pathway and its implications in coagulation, infections and auto-immunity. Curr Opin Organ Transplant. 10.1097/MOT.0b013e32834253df [doi]. - PubMed
- Thiel S (2007) Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol Immunol 44: 3875–3888. - PubMed
- Endo Y, Sato Y, Matsushita M, Fujita T (1996) Cloning and characterization of the human lectin P35 gene and its related gene. Genomics 36: 515–521. S0888-7543(96)90497-8 [pii];10.1006/geno.1996.0497 [doi]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous