G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness - PubMed (original) (raw)
. 2012 Dec 4;5(253):ra89.
doi: 10.1126/scisignal.2003264.
Oscar Vadas, Aliaksei Shymanets, John E Burke, Rachel S Salamon, Bassem D Khalil, Mathew O Barrett, Gary L Waldo, Chinmay Surve, Christine Hsueh, Olga Perisic, Christian Harteneck, Peter R Shepherd, T Kendall Harden, Alan V Smrcka, Ronald Taussig, Anne R Bresnick, Bernd Nürnberg, Roger L Williams, Jonathan M Backer
Affiliations
- PMID: 23211529
- PMCID: PMC3979326
- DOI: 10.1126/scisignal.2003264
G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness
Hashem A Dbouk et al. Sci Signal. 2012.
Abstract
Synergistic activation by heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors (GPCRs) and receptor tyrosine kinases distinguishes p110β from other class IA phosphoinositide 3-kinases (PI3Ks). Activation of p110β is specifically implicated in various physiological and pathophysiological processes, such as the growth of tumors deficient in phosphatase and tensin homolog deleted from chromosome 10 (PTEN). To determine the specific contribution of GPCR signaling to p110β-dependent functions, we identified the site in p110β that binds to the Gβγ subunit of G proteins. Mutation of this site eliminated Gβγ-dependent activation of PI3Kβ (a dimer of p110β and the p85 regulatory subunit) in vitro and in cells, without affecting basal activity or phosphotyrosine peptide-mediated activation. Disrupting the p110β-Gβγ interaction by mutation or with a cell-permeable peptide inhibitor blocked the transforming capacity of PI3Kβ in fibroblasts and reduced the proliferation, chemotaxis, and invasiveness of PTEN-null tumor cells in culture. Our data suggest that specifically targeting GPCR signaling to PI3Kβ could provide a therapeutic approach for tumors that depend on p110β for growth and metastasis.
Conflict of interest statement
Competing interests: H.A.D., R.S.S., B.D.K., and J.M.B. have a patent pending on the development of therapeutics targeting the p110β-Gβγ interface. P.R.S. is a founder scientist of Pathway Therapeutics.
Figures
Fig. 1
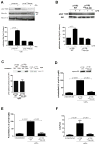
Mapping of the Gβγ-binding site on p110β by sequence analysis and HDX-MS. (A) Sequence alignment of the C2 domain–helical domain linker region of p110α, β, and δ. The black rectangles denote helices in the p110β structure, and the black line represents the disordered region. (B) Cartoon illustration of the p110β–p85α-icSH2 wild-type (WT) heterodimer and the p110β–Gβ-p85α-icSH2–Gγ-C68S fusion heterotrimer (fusion) used for the HDX-MS experiments. (C) Domains of p110β are outlined and colored according to the legend for changes associated with the presence of Gβγ. Regions in p110β and p85α-icSH2 that showed >0.5 Dalton and >5% changes in deuteration extent between the WT and fusion complexes were mapped on the p110β–p85β–icSH2 model (PDB: 2y3a, right panel). The loop region between the C2 domain and the helical domain is represented as a dotted line because it is not ordered in the structure. Residues corresponding to human p110β K532 and K533 are represented with balls and sticks. Top left, a close-up view of the p110β region in which changes in deuteration extent as a result of the presence of Gβγ were detected is shown. Bottom left, a model for the p110β–p85α-nicSH2 generated by combining the structures of p110β–p85β-icSH2 (PDB: 2Y3A) and p85α–nSH2 (PDB: 3HHM). The nSH2 and cSH2 domains of p85 are shown as surface representations. The p85α-nSH2 position is based on the structure of p110α, although there is no unambiguous evidence that nSH2 adopts exactly the same position when in complex with p110β. (D) Sequence of the loop-swap mutant of p110β. (E) Alignment of p110β zoologs in the region of the C2-helical linker. (F) Activities of WT PI3Kβ and the loop-swap and 532KK-DD mutants purified from insect cells, in the presence of pY peptide (pY) and lipidated Gβγ. Activities were expressed relative to the basal activity of PI3Kβ, which was normalized to one. Graph shows the activity ±SD of three independent experiments.
Fig. 2
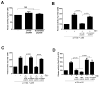
Role of Gβγ in PI3Kβ-mediated signaling, transformation, motility, and invasion. (A) HEK 293E cells were transfected with plasmids encoding myc-Akt and either WT or the 532KK-DD mutant PI3Kβ, with or without plasmids encoding Gβγ. Akt activation in samples immunoprecipitated (IP) with an antibody against myc was analyzed by Western blotting with an antibody against pT308-Akt. The ratio of the amount of pAkt to that of total Akt is expressed as a percentage of that under basal conditions. (B) NIH3T3 cells stably expressing WT or mutant PI3Kβ were stimulated with 10 nM LPA for 5 min. Akt activation was analyzed by Western blotting with anti-pT308-Akt antibody and quantified as described earlier. (C) HEK 293T cells were transfected with plasmid encoding WT or 532KK-DD mutant p110β. Cell lysates were incubated with GST or GST-Rab5 immobilized on glutathione-Sepharose beads, and bound material was analyzed by Western blotting. Graphs in each panel show the mean percentage pulldown ± SEM from three separate experiments. (D and E) NIH3T3 cells were transfected with plasmids encoding p85α and either WT or the 532KK-DD mutant p110β, and (D) the formation of colonies in soft agar or (E) the formation of foci were measured. Graphs in each panel show means ± SEM from three separate experiments. (F) Migration of control NIH3T3 cells or cells stably expressing WT or mutant PI3Kβ towards FBS was measured in a Boyden chamber assay. Assays were conducted in triplicate, and the data are pooled from two separate experiments.
Fig. 3
Mapping of the p110β-binding region in Gβγ heterodimers with HDX-MS. (A) The p110β–Gβ-p85α-icSH2–Gγ-C68S fusion heterotrimer (fusion) was used to compare deuterium incorporation with that of free Gβγ-C68S (Gβγ). Regions in Gβ and Gγ that showed >0.5 Dalton and >5% changes between free Gβγ and the fusion were mapped onto the Gβγ model (PDB ID: 1GOT). In addition to the protected peptides described in the text, there was some exposure of the C-terminus of Gβ and the adjacent C-terminus of Gγ, which were probably a consequence of the attachment of the C-terminus of Gβ to the linker connecting to p85 in the fusion. (B) All peptides in Gβ and Gγ that showed changes in deuteration extent between free Gβγ and the fusion proteins are shown. The stretch of amino acid residues 52 to 66 in Gγ is labeled as a segment to denote that these data were generated by subtraction of the deuterium incorporation of peptide 44 to 51 from that of peptide 44 to 66. * indicates changes that were >0.5 Dalton and >5%. Experiments were performed in duplicate and graphs show the SD. (C) Crystal structure of Gβγ bound to Gα (PDB ID:1GOT). Gβγ is colored as in (A).
Fig. 4
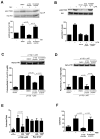
A peptide derived from p110β blocks the activation of PI3Kβ by G βγ in vitro. (A). PI3Kβ immunopurified from HEK 293T cells was incubated in the absence or presence of 1 μM p110β-peptide or scrambled peptide and assayed for lipid kinase activity. (B) PI3Kβ immunopurified from HEK 293T cells was incubated in the absence or presence of recombinant lipidated Gβγ and 1 μM p110β-peptide or scrambled peptide and assayed for lipid kinase activity. (C) PI3Kβ immunopurified from HEK 293T cells was incubated in the absence or presence of recombinant lipidated Gβγ and 1 μM myristoylated or TAT-tagged p110β-peptide or scrambled peptide and assayed for lipid kinase activity. (D) Immunopurified p101–p110γ from HEK 293T cells was incubated with or without recombinant lipidated Gβγ and 1 μM p110β-peptide, scrambled peptide, 1 μM QEHA peptide, or 10 μM SIGK peptide. Data in all panels are the means ± SEM of triplicate measurements and are representative of two to three experiments.
Fig. 5
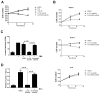
Peptide inhibitors disrupt PI3Kβ activation and signaling in response to Gβγ. (A) HEK 293E cells were transfected with plasmids encoding p110β, p85, and myc-Akt with or without plasmid encoding Gβγ. Cells were treated with 30 μM peptide or scrambled peptide for 30 min, and the extent of phosphorylation of Akt at Thr308 (T308) was determined by Western blotting analysis. (B) NIH3T3 cells were pretreated with TGX221, p110β-peptide, or scrambled peptide and stimulated with 10 nM LPA for 5 min before the extent of phosphorylation of Akt at Thr308 was determined by Western blotting analysis. (C) NIH3T3 cells were transfected with plasmids encoding WTp110β and p85α, and colony formation in soft agar was measured in the absence or presence of 30 μM p110β-derived myristoylated peptide or scrambled peptide. (D) NIH3T3 cells were transfected with plasmids encoding WT p110β and p85α, and foci formation was measured in the absence or presence of 30 μM p110β-derived myristoylated peptide or scrambled peptide. (E) NIH3T3 cells were transfected with plasmids encoding p110β and p85, or with plasmid encoding 12V-Ras. Cells were incubated with or without p110β-peptide or scrambled peptide, and formation of colonies in soft agar was measured. (F) Migration of NIH3T3 stably expressing p110β and p85α towards FBS in a Boyden chamber, in the absence or presence of p110 β-peptide or scrambled peptide. The graphs in each panel show means ± SEM from three to four separate experiments. Data in (E) show means ± SEM from triplicate measurements and are representative of two experiments.
Fig. 6
Inhibition of the proliferation and chemotaxis of prostate cancer cells. (A) Proliferation of PC-3 cells was measured by the MTT assay in the absence or presence of 200 nM TGX221, 30 μM myristoylated p110β-derived peptide, or 30 μM scrambled peptide. (B) Proliferation assays were performed on two PTEN-null endometrial cancer cell lines (AN3CA and RL95-2 cells) and one PTEN-positive endometrial cancer cell line (KLE cells) grown in the absence or presence of myristoylated p110β-derived peptide or scrambled peptide. (C) Chemotaxis of PC-3 cells towards 10% FBS in the absence or presence of 20 μM p110β-derived peptide or scrambled peptide was measured in Boyden chambers. (D) Bone marrow–derived macrophages and Cell-tracker-red–labeled PC-3 tumor cells were co-plated in 24-well dishes and overlaid with collagen. Cells were incubated for 24 hours in the absence or presence of p110β-derived peptide or scrambled peptide, and invasion into the collagen was measured by confocal microscopy. The data are means ± SD from two separate experiments for (B) and (D), and are means ± SEM from three separate experiments for (A) and (C).
Similar articles
- Rac1-stimulated macropinocytosis enhances Gβγ activation of PI3Kβ.
Erami Z, Khalil BD, Salloum G, Yao Y, LoPiccolo J, Shymanets A, Nürnberg B, Bresnick AR, Backer JM. Erami Z, et al. Biochem J. 2017 Nov 16;474(23):3903-3914. doi: 10.1042/BCJ20170279. Biochem J. 2017. PMID: 29046393 Free PMC article. - Coincident signals from GPCRs and receptor tyrosine kinases are uniquely transduced by PI3Kβ in myeloid cells.
Houslay DM, Anderson KE, Chessa T, Kulkarni S, Fritsch R, Downward J, Backer JM, Stephens LR, Hawkins PT. Houslay DM, et al. Sci Signal. 2016 Aug 16;9(441):ra82. doi: 10.1126/scisignal.aae0453. Sci Signal. 2016. PMID: 27531651 Free PMC article. - A function for phosphoinositide 3-kinase beta lipid products in coupling beta gamma to Ras activation in response to lysophosphatidic acid.
Yart A, Roche S, Wetzker R, Laffargue M, Tonks N, Mayeux P, Chap H, Raynal P. Yart A, et al. J Biol Chem. 2002 Jun 14;277(24):21167-78. doi: 10.1074/jbc.M110411200. Epub 2002 Mar 26. J Biol Chem. 2002. PMID: 11916960 - Gβγ Pathways in Cell Polarity and Migration Linked to Oncogenic GPCR Signaling: Potential Relevance in Tumor Microenvironment.
Vázquez-Prado J, Bracho-Valdés I, Cervantes-Villagrana RD, Reyes-Cruz G. Vázquez-Prado J, et al. Mol Pharmacol. 2016 Nov;90(5):573-586. doi: 10.1124/mol.116.105338. Epub 2016 Sep 16. Mol Pharmacol. 2016. PMID: 27638873 Review. - Taking the heart failure battle inside the cell: small molecule targeting of Gβγ subunits.
Kamal FA, Smrcka AV, Blaxall BC. Kamal FA, et al. J Mol Cell Cardiol. 2011 Oct;51(4):462-7. doi: 10.1016/j.yjmcc.2011.01.006. Epub 2011 Jan 21. J Mol Cell Cardiol. 2011. PMID: 21256851 Free PMC article. Review.
Cited by
- Aloe emodin promotes mucosal healing by modifying the differentiation fate of enteroendocrine cells via regulating cellular free fatty acid sensitivity.
Bao W, Lyu J, Feng G, Guo L, Zhao D, You K, Liu Y, Li H, Du P, Chen D, Shen X. Bao W, et al. Acta Pharm Sin B. 2024 Sep;14(9):3964-3982. doi: 10.1016/j.apsb.2024.05.027. Epub 2024 May 31. Acta Pharm Sin B. 2024. PMID: 39309505 Free PMC article. - A novel model based on protein post-translational modifications comprising the immune landscape and prediction of colorectal cancer prognosis.
Yu T, Yan J, Liu C, Yao C, Xu Y, Xu J, Xu J, Sun Q. Yu T, et al. J Gastrointest Oncol. 2024 Aug 31;15(4):1592-1612. doi: 10.21037/jgo-24-45. Epub 2024 Jul 16. J Gastrointest Oncol. 2024. PMID: 39279963 Free PMC article. - Molecular dissection of PI3Kβ synergistic activation by receptor tyrosine kinases, GβGγ, and Rho-family GTPases.
Duewell BR, Wilson NE, Bailey GM, Peabody SE, Hansen SD. Duewell BR, et al. Elife. 2024 May 7;12:RP88991. doi: 10.7554/eLife.88991. Elife. 2024. PMID: 38713746 Free PMC article. - Molecular basis for differential activation of p101 and p84 complexes of PI3Kγ by Ras and GPCRs.
Rathinaswamy MK, Jenkins ML, Duewell BR, Zhang X, Harris NJ, Evans JT, Stariha JTB, Dalwadi U, Fleming KD, Ranga-Prasad H, Yip CK, Williams RL, Hansen SD, Burke JE. Rathinaswamy MK, et al. Cell Rep. 2023 Mar 28;42(3):112172. doi: 10.1016/j.celrep.2023.112172. Epub 2023 Feb 26. Cell Rep. 2023. PMID: 36842083 Free PMC article. - The spatial distribution of GPCR and Gβγ activity across a cell dictates PIP3 dynamics.
Wijayaratna D, Ratnayake K, Ubeysinghe S, Kankanamge D, Tennakoon M, Karunarathne A. Wijayaratna D, et al. Sci Rep. 2023 Feb 16;13(1):2771. doi: 10.1038/s41598-023-29639-0. Sci Rep. 2023. PMID: 36797332 Free PMC article.
References
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. - PubMed
- Kulkarni S, Sitaru C, Jakus Z, Anderson KE, Damoulakis G, Davidson K, Hirose M, Juss J, Oxley D, Chessa TA, Ramadani F, Guillou H, Segonds-Pichon A, Fritsch A, Jarvis GE, Okkenhaug K, Ludwig R, Zillikens D, Mocsai A, Vanhaesebroeck B, Stephens LR, Hawkins PT. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011;4:ra23. - PubMed
- Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βgamma subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA100324/CA/NCI NIH HHS/United States
- F31 AG040932/AG/NIA NIH HHS/United States
- R01 GM055692/GM/NIGMS NIH HHS/United States
- R01 GM057391/GM/NIGMS NIH HHS/United States
- GM55692/GM/NIGMS NIH HHS/United States
- PG/11/109/29247/BHF_/British Heart Foundation/United Kingdom
- R01 GM081772/GM/NIGMS NIH HHS/United States
- PG11/109/29247/BHF_/British Heart Foundation/United Kingdom
- U105184308/MRC_/Medical Research Council/United Kingdom
- T32 GM007491/GM/NIGMS NIH HHS/United States
- GM57391/GM/NIGMS NIH HHS/United States
- MC_U105184308/MRC_/Medical Research Council/United Kingdom
- 5T32 GM007491/GM/NIGMS NIH HHS/United States
- 1 F31 AG040932-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials