Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model - PubMed (original) (raw)
Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model
Chunting Ye et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Hypersecretion of cytokines by innate immune cells is thought to initiate multiple organ failure in murine models of sepsis. Whether human cytokine storm also plays a similar role is not clear. Here, we show that human hematopoietic cells are required to induce sepsis-induced mortality following cecal ligation and puncture (CLP) in the severely immunodeficient nonobese diabetic (NOD)/SCID/IL2Rγ(-/-) mice, and siRNA treatment to inhibit HMGB1 release by human macrophages and dendritic cells dramatically reduces sepsis-induced mortality. Following CLP, compared with immunocompetent WT mice, NOD/SCID/IL2Rγ(-/-) mice did not show high levels of serum HMGB1 or murine proinflammatory cytokines and were relatively resistant to sepsis-induced mortality. In contrast, NOD/SCID/IL2Rγ(-/-) mice transplanted with human hematopoietic stem cells [humanized bone marrow liver thymic mice (BLT) mice] showed high serum levels of HMGB1, as well as multiple human but not murine proinflammatory cytokines, and died uniformly, suggesting human cytokines are sufficient to induce organ failure in this model. Moreover, targeted delivery of HMGB1 siRNA to human macrophages and dendritic cells using a short acetylcholine receptor (AchR)-binding peptide [rabies virus glycoprotein (RVG)-9R] effectively suppressed secretion of HMGB1, reduced the human cytokine storm, human lymphocyte apoptosis, and rescued humanized mice from CLP-induced mortality. siRNA treatment was also effective when started after the appearance of sepsis symptoms. These results show that CLP in humanized mice provides a model to study human sepsis, HMGB1 siRNA might provide a treatment strategy for human sepsis, and RVG-9R provides a tool to deliver siRNA to human macrophages and dendritic cells that could potentially be used to suppress a variety of human inflammatory diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
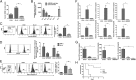
Human hematopoietic cells are required to induce sepsis in NOD/SCID/IL2Rγ−/− mice. Mice underwent sham or CLP surgery and 24 h post-CLP, sera were tested for HMGB1 by ELISA (A), murine proinflammatory cytokines were tested by cytometric bead array (B), and human proinflammatory cytokines were tested by cytometric bead array (C). Mean ± SD from seven mice per group is shown. (D) Kaplan–Meier survival curves for mice following CLP are shown (n = 14 per group). (E) Representative hematoxylin and eosin (H&E)-stained sections from the liver taken 24 h after CLP from the indicated group of mice are shown. (F) Representative TUNEL-stained liver section (Left) and cumulative percentage of TUNEL+ cells determined by examining 20 high-powered fields from three mice in each group (Right) are shown. (G) Bacterial counts in the blood of indicated groups of mice on indicated days after CLP is shown (n = 5–7 per group). BALB/c, BLT, and neutrophil-depleted NOD/SCID/IL2Rγ−/− mice could not be serially followed because all mice died by day 2–3. (H) Neutrophil depletion was tested in blood 3 d after administration of Ly6G-specific mAb by staining for Ly6G and GR-1. (I) Kaplan–Meier survival curves for NOD/SCID/IL2Rγ−/− mice with intact or depleted neutrophils is shown (n = 5 per group). *P < 0.05.
Fig. 2.
RVG-9R delivers siRNA to primary human macrophages and DCs. (A) Ex vivo–isolated human PBMCs were incubated with FITC siLuci complexed with RVG-9R for 4 h before staining with antibodies to AchR, CD14, CD11c, CD3, and CD19. CD14-, CD11c-, CD3-, and CD19-gated cell populations were examined for FITC uptake by flow cytometry. The experiment was repeated two times with similar results. (B) In vitro–cultured human macrophages, DCs, and T-cell blasts were treated with FITC siLuci alone, transfected with FITC siLuci using Lipofectamine 2000 or transduced with FITC siLuci complexed with RVG-9R or a control RV-MAT-9R peptide, and cells were examined for FITC siLuci uptake by flow cytometry. The experiment was repeated two times with similar results. (C) Cultured macrophages and DCs were transfected/transduced as in B with siRNA targeting cyclophilin B and, after 24 h, tested for cyclophilin B mRNA levels by qRT-PCR. Mean ± SD of triplicates is shown. (D) Splenocytes from humanized mice were examined for FITC siRNA transduction as in A. (E) BLT mice were injected (i.v.) with either control Luci siRNA complexed with RVG-9R or TNF-α siRNA complexed with RVG-9R or a control RV-MAT-9R peptide 18 and 6 h before LPS injection (i.p.). One hour after LPS injection, human TNF-α mRNA levels in PBMCs were tested by qRT-PCR, and serum human TNF-α protein levels were tested by ELISA (Center and Right). Human CD14+ cells in PBMCs of the three groups of mice before the start of the experiment are shown in the left panel. Each symbol represents an individual mouse. *P < 0.05.
Fig. 3.
Treatment of humanized mice with HMGB1 siRNA/RVG-9R suppresses CLP-induced sepsis. Humanized mice were treated i.v. with either a control Luci siRNA or HMGB1 siRNA complexed with RVG-9R, as detailed in
SI Materials and Methods
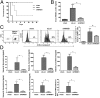
, before and after CLP. Sham indicates sham surgery without CLP or siRNA treatment. (A) Sera obtained 20 h post-CLP were tested for HMGB1 protein levels by ELISA. Mean ± SD from seven mice per group is shown. (B) Bacterial counts in the blood of control and siRNA treated mice at indicated times after CLP are shown. Control mice could not be serially followed because they all died by day 3. (C) Twenty hours post-CLP, splenocytes were tested for HMGB1 expression in human CD45+, CD14+ gated cells by flow cytometry. (Left and Right) Representative flow-cytometric results (Left) and cumulative data from seven mice per group ± SD (Right) are shown. (D) CD14+ cells isolated from control and siRNA-treated mice were tested for TNF-α and IL-1β levels by qRT-PCR (n = 5 per group). (Left) Purity of isolated cells. (E) PBMCs obtained 20 h post-CLP were tested for active caspase-3+ cells within human CD45+ cells by flow cytometry. (Left and Right) Representative flow-cytometric result (Left) and cumulative data from seven mice per group (Right) are shown. (F) Sera obtained 20 h post-CLP were tested for indicated human cytokines by cytometric bead array. Mean ± SD from seven mice per group is shown. (G) Sera obtained 20 h post-CLP were tested for indicated murine cytokines by cytometric bead array. Mean ± SD from seven mice per group is shown. (H) Kaplan–Meier survival curves for mice following CLP is shown (n = 14 per group).
Fig. 4.
Post-CLP treatment with HMGB1 siRNA/RVG-9R also affords protection. (A) CLP was performed, and siRNA treatment, as detailed in the legend of Fig. 3, was started 10 h later, and mice were followed for survival. (B_–_D) Twenty-four hours after CLP, mice were tested for serum HMGB1 levels (B), active caspase-3 positivity in PBMCs (C), and serum human cytokine levels (D), as described in the legend of Fig. 3 (n = 7 mice per group).
Similar articles
- Penehyclidine hydrochloride inhibits the release of high-mobility group box 1 in lipopolysaccharide-activated RAW264.7 cells and cecal ligation and puncture-induced septic mice.
Yang Q, Liu X, Yao Z, Mao S, Wei Q, Chang Y. Yang Q, et al. J Surg Res. 2014 Jan;186(1):310-7. doi: 10.1016/j.jss.2013.08.015. Epub 2013 Sep 8. J Surg Res. 2014. PMID: 24124976 - Anti-Septic Functions of Cornuside against HMGB1-Mediated Severe Inflammatory Responses.
Kim N, Kim C, Ryu SH, Lee W, Bae JS. Kim N, et al. Int J Mol Sci. 2022 Feb 13;23(4):2065. doi: 10.3390/ijms23042065. Int J Mol Sci. 2022. PMID: 35216180 Free PMC article. - Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production.
Subramanya S, Kim SS, Abraham S, Yao J, Kumar M, Kumar P, Haridas V, Lee SK, Shultz LD, Greiner D, N M, Shankar P. Subramanya S, et al. J Virol. 2010 Mar;84(5):2490-501. doi: 10.1128/JVI.02105-08. Epub 2009 Dec 16. J Virol. 2010. PMID: 20015996 Free PMC article. - [Role of alarmins high mobility group protein B1 in sepsis].
Wei J, Zhang Y, Ma X, Tian L, Liang H. Wei J, et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016 Aug;28(8):761-4. doi: 10.3760/cma.j.issn.2095-4352.2016.08.021. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016. PMID: 27434574 Review. Chinese. - Potential effects of HMGB1 on viral replication and virus infection-induced inflammatory responses: A promising therapeutic target for virus infection-induced inflammatory diseases.
Ding X, Li S, Zhu L. Ding X, et al. Cytokine Growth Factor Rev. 2021 Dec;62:54-61. doi: 10.1016/j.cytogfr.2021.08.003. Epub 2021 Sep 1. Cytokine Growth Factor Rev. 2021. PMID: 34503914 Review.
Cited by
- The Fluctuations of Leukocytes and Circulating Cytokines in Septic Humanized Mice Vary With Outcome.
Skirecki T, Drechsler S, Hoser G, Jafarmadar M, Siennicka K, Pojda Z, Kawiak J, Osuchowski MF. Skirecki T, et al. Front Immunol. 2019 Jun 26;10:1427. doi: 10.3389/fimmu.2019.01427. eCollection 2019. Front Immunol. 2019. PMID: 31297113 Free PMC article. - HMGB1 as a therapeutic target in disease.
Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI, Carbone M, Yang H. Xue J, et al. J Cell Physiol. 2021 May;236(5):3406-3419. doi: 10.1002/jcp.30125. Epub 2020 Oct 26. J Cell Physiol. 2021. PMID: 33107103 Free PMC article. Review. - Potential Pitfalls of the Humanized Mice in Modeling Sepsis.
Laudanski K, Stentz M, DiMeglio M, Furey W, Steinberg T, Patel A. Laudanski K, et al. Int J Inflam. 2018 Sep 2;2018:6563454. doi: 10.1155/2018/6563454. eCollection 2018. Int J Inflam. 2018. PMID: 30245803 Free PMC article. Review. - Humanizing the mouse: in defense of murine models of critical illness.
Perlman H, Budinger GR, Ward PA. Perlman H, et al. Am J Respir Crit Care Med. 2013 May 1;187(9):898-900. doi: 10.1164/rccm.201303-0489ED. Am J Respir Crit Care Med. 2013. PMID: 23634853 Free PMC article. No abstract available. - Time to Develop Therapeutic Antibodies Against Harmless Proteins Colluding with Sepsis Mediators?
Li J, Bao G, Wang H. Li J, et al. Immunotargets Ther. 2020 Oct 5;9:157-166. doi: 10.2147/ITT.S262605. eCollection 2020. Immunotargets Ther. 2020. PMID: 33117741 Free PMC article. Review.
References
- Antonopoulou A, Giamarellos-Bourboulis EJ. Immunomodulation in sepsis: State of the art and future perspective. Immunotherapy. 2011;3(1):117–128. - PubMed
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. - PubMed
- Skirecki T, Borkowska-Zielinska U, Zlotorowicz M, Hoser G. Sepsis immunopathology: Perspectives of monitoring and modulation of the immune disturbances. Arch Immunol Ther Exp (Warsz) 2012;60(2):123–135. - PubMed
- Abraham E, et al. Lenercept Study Group Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: A randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29(3):503–510. - PubMed
- Abraham E, et al. NORASEPT II Study Group Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet. 1998;351(9107):929–933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous