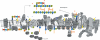Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall - PubMed (original) (raw)
Review
Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall
Arnold S Bayer et al. Ann N Y Acad Sci. 2013 Jan.
Abstract
The bactericidal, cell membrane-targeting lipopeptide antibiotic daptomycin (DAP) is an important agent in treating invasive Staphylococcus aureus infections. However, there have been numerous recent reports of development of daptomycin resistance (DAP-R) during therapy with this agent. The mechanisms of DAP-R in S. aureus appear to be quite diverse. DAP-R strains often exhibit progressive accumulation of single nucleotide polymorphisms in the multipeptide resistance factor gene (mprF) and the yycFG components of the yycFGHI operon. Both loci are involved in key cell membrane (CM) events, with mprF being responsible for the synthesis and outer CM translocation of the positively charged phospholipid, lysyl-phosphotidylglycerol (L-PG), while the yyc operon is involved in the generalized response to stressors such as antimicrobials. In addition, other perturbations of the CM have been identified in DAP-R strains, including extremes in CM order, resistance to CM depolarization and permeabilization, and reduced surface binding of DAP. Moreover, modifications of the cell wall (CW) appear to also contribute to DAP-R, including enhanced expression of the dlt operon (involved in d-alanylation of CW teichoic acids) and progressive CW thickening.
© 2012 New York Academy of Sciences.
Figures
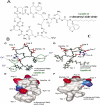
Figure 1
Chemical structure of calcium-DAP based on NMR analyses. (A) Basic chemical structure of the entire lipo-peptide DAP molecule; (B) and (C) Model of the apostructure and calcium-conjugated structure, respectively. Negatively charged side chains are colored red, while positively charged side chains are colored blue. (E) and (F) Surface representation of the apostructure and calcium-conjugated structure of DAP, respectively, with red representing negative charges, blue representing positive charges, and white representing uncharged regions. Modified from data in Refs. 27–30a.
Figure 2
Proposed tri-domain structure-function topology of the MprF molecule by TOPCONS algorithm construction (i.e., C-terminal synthase domain; N-terminal flippase domain; and central bifunctional domains). The sites and amino acid modifications of the five SNPs most commonly observed in association with DAP-R are represented by the star-burst symbols. Modified from Ernst et al. (Reproduced with permission of C. Ernst and A. Peschel).
Figure 3
(A) Cartoon of enzymes and bactroprenol-bound substrates comprising the partially overlapping machineries for biosynthesis of CW teichoic acids (left), peptidoglycan (middle) and for cell division (right). CM areas in which these pathways take place are rich negatively-charged bactoprenol-phosphate/pyrophosphate and PG (indicated in yellow), and thus attract DAP and other CAPs. (B) Biosynthetic pathways generating negatively-charges lipids and CW components, and reactions involved in modulation of the surface charge by D-alanylation and lysinylation (positive charges indicated in red) resulting in reduced DAP activity and DAP-R development. (C) Sensor systems involved in controlling CW integrity and proposed regulons; mutations in yycG (vicK) and vraS frequently observed in DAP-R mutants may reduce precision in CW structure and function, and allow for growth of impaired, but viable cells.
Figure 3
(A) Cartoon of enzymes and bactroprenol-bound substrates comprising the partially overlapping machineries for biosynthesis of CW teichoic acids (left), peptidoglycan (middle) and for cell division (right). CM areas in which these pathways take place are rich negatively-charged bactoprenol-phosphate/pyrophosphate and PG (indicated in yellow), and thus attract DAP and other CAPs. (B) Biosynthetic pathways generating negatively-charges lipids and CW components, and reactions involved in modulation of the surface charge by D-alanylation and lysinylation (positive charges indicated in red) resulting in reduced DAP activity and DAP-R development. (C) Sensor systems involved in controlling CW integrity and proposed regulons; mutations in yycG (vicK) and vraS frequently observed in DAP-R mutants may reduce precision in CW structure and function, and allow for growth of impaired, but viable cells.
Figure 3
(A) Cartoon of enzymes and bactroprenol-bound substrates comprising the partially overlapping machineries for biosynthesis of CW teichoic acids (left), peptidoglycan (middle) and for cell division (right). CM areas in which these pathways take place are rich negatively-charged bactoprenol-phosphate/pyrophosphate and PG (indicated in yellow), and thus attract DAP and other CAPs. (B) Biosynthetic pathways generating negatively-charges lipids and CW components, and reactions involved in modulation of the surface charge by D-alanylation and lysinylation (positive charges indicated in red) resulting in reduced DAP activity and DAP-R development. (C) Sensor systems involved in controlling CW integrity and proposed regulons; mutations in yycG (vicK) and vraS frequently observed in DAP-R mutants may reduce precision in CW structure and function, and allow for growth of impaired, but viable cells.
Similar articles
- Phenotypic and genotypic correlates of daptomycin-resistant methicillin-susceptible Staphylococcus aureus clinical isolates.
Kang KM, Mishra NN, Park KT, Lee GY, Park YH, Bayer AS, Yang SJ. Kang KM, et al. J Microbiol. 2017 Feb;55(2):153-159. doi: 10.1007/s12275-017-6509-1. Epub 2017 Jan 26. J Microbiol. 2017. PMID: 28120188 Free PMC article. - Emergence of daptomycin resistance in daptomycin-naïve rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms.
Mishra NN, Yang SJ, Chen L, Muller C, Saleh-Mghir A, Kuhn S, Peschel A, Yeaman MR, Nast CC, Kreiswirth BN, Crémieux AC, Bayer AS. Mishra NN, et al. PLoS One. 2013 Aug 19;8(8):e71151. doi: 10.1371/journal.pone.0071151. eCollection 2013. PLoS One. 2013. PMID: 23990934 Free PMC article. - Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates.
Bertsche U, Yang SJ, Kuehner D, Wanner S, Mishra NN, Roth T, Nega M, Schneider A, Mayer C, Grau T, Bayer AS, Weidenmaier C. Bertsche U, et al. PLoS One. 2013 Jun 13;8(6):e67398. doi: 10.1371/journal.pone.0067398. Print 2013. PLoS One. 2013. PMID: 23785522 Free PMC article. - Mechanisms of resistance to daptomycin in Staphylococcus aureus.
Gómez Casanova N, Siller Ruiz M, Muñoz Bellido JL. Gómez Casanova N, et al. Rev Esp Quimioter. 2017 Dec;30(6):391-396. Epub 2017 Oct 25. Rev Esp Quimioter. 2017. PMID: 29082727 Review. - Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence.
Stefani S, Campanile F, Santagati M, Mezzatesta ML, Cafiso V, Pacini G. Stefani S, et al. Int J Antimicrob Agents. 2015 Sep;46(3):278-89. doi: 10.1016/j.ijantimicag.2015.05.008. Epub 2015 Jun 19. Int J Antimicrob Agents. 2015. PMID: 26143590 Review.
Cited by
- Strain Dependent Genetic Networks for Antibiotic-Sensitivity in a Bacterial Pathogen with a Large Pan-Genome.
van Opijnen T, Dedrick S, Bento J. van Opijnen T, et al. PLoS Pathog. 2016 Sep 8;12(9):e1005869. doi: 10.1371/journal.ppat.1005869. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27607357 Free PMC article. - Therapeutic potential of the antimicrobial peptide OH-CATH30 for antibiotic-resistant Pseudomonas aeruginosa keratitis.
Li SA, Liu J, Xiang Y, Wang YJ, Lee WH, Zhang Y. Li SA, et al. Antimicrob Agents Chemother. 2014 Jun;58(6):3144-50. doi: 10.1128/AAC.00095-14. Epub 2014 Mar 17. Antimicrob Agents Chemother. 2014. PMID: 24637683 Free PMC article. - In Vitro Activities of Novel Antimicrobial Combinations against Extensively Drug-Resistant Acinetobacter baumannii.
Córdoba J, Coronado-Álvarez NM, Parra D, Parra-Ruiz J. Córdoba J, et al. Antimicrob Agents Chemother. 2015 Dec;59(12):7316-9. doi: 10.1128/AAC.00493-15. Epub 2015 Sep 14. Antimicrob Agents Chemother. 2015. PMID: 26369956 Free PMC article. - Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids.
Tran TT, Panesso D, Mishra NN, Mileykovskaya E, Guan Z, Munita JM, Reyes J, Diaz L, Weinstock GM, Murray BE, Shamoo Y, Dowhan W, Bayer AS, Arias CA. Tran TT, et al. mBio. 2013 Jul 23;4(4):e00281-13. doi: 10.1128/mBio.00281-13. mBio. 2013. PMID: 23882013 Free PMC article. - Small cationic antimicrobial peptides delocalize peripheral membrane proteins.
Wenzel M, Chiriac AI, Otto A, Zweytick D, May C, Schumacher C, Gust R, Albada HB, Penkova M, Krämer U, Erdmann R, Metzler-Nolte N, Straus SK, Bremer E, Becher D, Brötz-Oesterhelt H, Sahl HG, Bandow JE. Wenzel M, et al. Proc Natl Acad Sci U S A. 2014 Apr 8;111(14):E1409-18. doi: 10.1073/pnas.1319900111. Epub 2014 Mar 24. Proc Natl Acad Sci U S A. 2014. PMID: 24706874 Free PMC article.
References
- Liu C, Bayer A, Cosgrove SE, et al. Infectious Diseases Society of America (IDSA) Clinical practice guidelines by the IDSA for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. - PubMed
- Vancomycin-resistant Staphylococcus aureus – Pennsylvania. MMWR (Morb Mortal Wkly Rep. 2002;51:565–567. 2002. - PubMed
- Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco 2004–2005. Clin Infect Dis. 2008;46:1637–1646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical