The ubiquitin proteasome system and myocardial ischemia - PubMed (original) (raw)
Review
The ubiquitin proteasome system and myocardial ischemia
Justine Calise et al. Am J Physiol Heart Circ Physiol. 2013.
Abstract
The ubiquitin proteasome system (UPS) has been the subject of intensive research over the past 20 years to define its role in normal physiology and in pathophysiology. Many of these studies have focused in on the cardiovascular system and have determined that the UPS becomes dysfunctional in several pathologies such as familial and idiopathic cardiomyopathies, atherosclerosis, and myocardial ischemia. This review presents a synopsis of the literature as it relates to the role of the UPS in myocardial ischemia. Studies have shown that the UPS is dysfunctional during myocardial ischemia, and recent studies have shed some light on possible mechanisms. Other studies have defined a role for the UPS in ischemic preconditioning which is best associated with myocardial ischemia and is thus presented here. Very recent studies have started to define roles for specific proteasome subunits and components of the ubiquitination machinery in various aspects of myocardial ischemia. Lastly, despite the evidence linking myocardial ischemia and proteasome dysfunction, there are continuing suggestions that proteasome inhibitors may be useful to mitigate ischemic injury. This review presents the rationale behind this and discusses both supportive and nonsupportive studies and presents possible future directions that may help in clarifying this controversy.
Figures
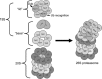
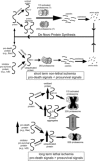
Fig. 1.
Proteasome structure. The 20S proteasome is a barrel-shaped structure composed of 4 rings, each consisting of 7 subunits. This 20S proteasome has immunoforms of the β2 (β2_i_) subunit in the upper β-ring and the β5 (β5_i_) subunit in the lower β-ring, illustrating the heterogeneity of this structure. Docked at one or both ends is the 19S regulatory particle. For the sake of clarity, the illustrated structure has only one 19S regulatory particle docked, known as the mushroom configuration and is actually 26S. Docking of two 19S regulatory particles at either end is called the dumbbell configuration and is actually 30S but is also commonly called the 26S proteasome. Rpn, regulatory particle non-ATPase subunit; Rpt, regulatory peptide triple A subunit; Ub, ubiquitin. Modified from Powell and Divald (110) with permission from Oxford University Press.
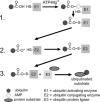
Fig. 2.
Ubiquitination of a protein. In step 1, a ubiquitin-activating enzyme (E1) binds ATP·Mg2+ and ubiquitin and then catalyzes ubiquitin COOH-terminus adenylation followed by attack of the catalytic E1-Cys on the ubiquitin-adenylate complex to form the activated E1-ubiquitin thioester complex. In step 2, ubiqutin is transferred to a ubiquitin-conjugating protein E2, producing a high-energy E2-ubiquitin thiol ester intermediate. In step 3, the intermediate is ligated to a protein substrate bound to a specific ubiquitin protein ligase E3. Modified from Powell and Divald (110) with permission from Oxford University Press.
Fig. 3.
The 11S-activated proteasome and the related hybrid proteasome. 11S-activated proteasomes are formed when an 11S activator ring docks at one or both ends of a 20S proteasome. The 11S activator ring is composed of either 3 protease activator (PA) 28α and 3 PA28β subunits (shown) or 3 PA28α and 4 PA28β subunits and may be induced by interferon-γ. One form of an 11S-activated proteasome has been called the immunoproteasome and contains one or more of the immunoforms of the catalytic β-type subunits. Another form of an 11S-activated proteasome does not contain β-subunit immunoforms. Also shown is the hybrid proteasome which has a 11S activator ring docked at one end of the 20S proteasome and a 19S regulatory particle docked at the end that, if it contain β-subunit immunoforms (shown), has also been called the immunoproteasome. Modified from Powell and Divald (110) with permission from Oxford University Press.
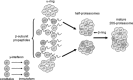
Fig. 4.
Regulation of 20S proteasomes by assembly of heterogeneous configurations. Under the influence of γ-interferon, select β-subunits are replaced by their immunoforms, which are directed by the complete α-rings to form half-proteasomes with specific configurations. In the example shown above, the top half-proteasome has constitutive β2 and β5 subunits and the β1 subunit has been replaced by the immunoform. The bottom half-proteasome has a constitutive β1 subunit, but the β2 and β5 subunits have been replaced by their immunoforms. These two distinctly different half-proteasomes mate to form a mature 20S proteasome that has a heterogeneous configuration. For the sake of clarity, the involvement of the proteasome assembly chaperones PAC1, PAC2, PAC3, and PAC4 and the proteasome maturation protein factor POMP are not depicted here. Modified from Powell (107) with the permission of Walters Kluwer Health.
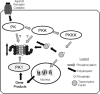
Fig. 5.
Regulation of proteasome activity by kinase cascades. In this scenario, binding of an agonist with a cell surface receptor initiates a phosphorylation cascade with an end effector protein kinase kinase kinase (PKKK) phosphorylating some transcription factor that can then enter the nucleus and interact with DNA resulting in gene transcription. Phosphorylation of signaling intermediates, in this case the transcription factor, is often a signal for ubiquitination and targeting to the 26S-proteasome for degradation. To amplify or dampen the effect of the signaling intermediate, any one of the protein kinases (PKs) within in the cascade might phosphorylate proteasome subunits, resulting in increased or decreased peptidase activity that would lessen or enhance availability of the signaling molecule accordingly. Another possibility would be for the gene product to activate a different protein kinase (PK1), which would phosphorylate proteasome resulting in activation and thus decreased availability of the transcription factor, essentially a form of feedback control. Reproduced from Powell (107) with the permission of Walters Kluwer Health.
Fig. 6.
Potential roles for the ubiquitin proteasome system (UPS) and 11S-activated proteasome in short- and long-duration ischemia. In the nonischemic heart, oxidized, misfolded, and ubiquitinated proteins are degraded through both ubiquitin- and nonubiquitin-mediated pathways, recycling the constituent amino acids and maintaining a dynamic balance between prosurvival and prodeath signals. During an ischemic insult resulting in cell death or dysfunction, UPS function is inhibited leading to accumulation of oxidized and ubiquitinated proteins. In addition, a condition known as dysregulation may occur in which normal UPS-mediated degradation of prodeath proteins is depressed. Reproduced from Powell and Divald (110) with permission from Oxford University Press.
Similar articles
- Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits.
Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, Gomes AV, Powell SR. Divald A, et al. Circ Res. 2010 Jun 25;106(12):1829-38. doi: 10.1161/CIRCRESAHA.110.219485. Epub 2010 Apr 29. Circ Res. 2010. PMID: 20431057 - The ubiquitin-proteasome system in myocardial ischaemia and preconditioning.
Powell SR, Divald A. Powell SR, et al. Cardiovasc Res. 2010 Jan 15;85(2):303-11. doi: 10.1093/cvr/cvp321. Epub 2009 Sep 30. Cardiovasc Res. 2010. PMID: 19793765 Free PMC article. Review. - The ubiquitin-proteasome system and cardiovascular disease.
Powell SR, Herrmann J, Lerman A, Patterson C, Wang X. Powell SR, et al. Prog Mol Biol Transl Sci. 2012;109:295-346. doi: 10.1016/B978-0-12-397863-9.00009-2. Prog Mol Biol Transl Sci. 2012. PMID: 22727426 Free PMC article. Review. - Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease.
Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Willis MS, et al. Circ Res. 2010 Feb 19;106(3):463-78. doi: 10.1161/CIRCRESAHA.109.208801. Circ Res. 2010. PMID: 20167943 Free PMC article. Review. - The ubiquitin proteasome system in human cardiomyopathies and heart failure.
Day SM. Day SM. Am J Physiol Heart Circ Physiol. 2013 May 15;304(10):H1283-93. doi: 10.1152/ajpheart.00249.2012. Epub 2013 Mar 11. Am J Physiol Heart Circ Physiol. 2013. PMID: 23479263 Free PMC article. Review.
Cited by
- A critical reappraisal of vasopressin and steroids in in-hospital cardiac arrest.
Mentzelopoulos SD, Chalkias A. Mentzelopoulos SD, et al. Crit Care. 2024 Jun 6;28(1):191. doi: 10.1186/s13054-024-04962-8. Crit Care. 2024. PMID: 38844995 Free PMC article. No abstract available. - Effects of canagliflozin on myocardial microvascular density, oxidative stress, and proteomic profile☆.
Sabe SA, Xu CM, Sabra M, Harris DD, Broadwin M, Bellam KG, Banerjee D, Usheva A, Ruhul Abid M, Sellke FW. Sabe SA, et al. J Mol Cell Cardiol Plus. 2023 Dec;6:100052. doi: 10.1016/j.jmccpl.2023.100052. Epub 2023 Sep 22. J Mol Cell Cardiol Plus. 2023. PMID: 38188970 Free PMC article. - Myocardial Protection and Current Cancer Therapy: Two Opposite Targets with Inevitable Cost.
Efentakis P, Andreadou I, Iliodromitis KE, Triposkiadis F, Ferdinandy P, Schulz R, Iliodromitis EK. Efentakis P, et al. Int J Mol Sci. 2022 Nov 15;23(22):14121. doi: 10.3390/ijms232214121. Int J Mol Sci. 2022. PMID: 36430599 Free PMC article. Review. - TIPE2 protects cardiomyocytes from ischemia-reperfusion-induced apoptosis by decreasing cell autophagy via the mTORC1 signaling pathway.
Cheng G, Huang X, You P, Feng P, Jia S, Zhang J, You H, Chang F. Cheng G, et al. Exp Ther Med. 2022 Aug 4;24(4):613. doi: 10.3892/etm.2022.11550. eCollection 2022 Oct. Exp Ther Med. 2022. PMID: 36160908 Free PMC article. - Physiologic effects of stress dose corticosteroids in in-hospital cardiac arrest (CORTICA): A randomized clinical trial.
Mentzelopoulos SD, Pappa E, Malachias S, Vrettou CS, Giannopoulos A, Karlis G, Adamos G, Pantazopoulos I, Megalou A, Louvaris Z, Karavana V, Aggelopoulos E, Agaliotis G, Papadaki M, Baladima A, Lasithiotaki I, Lagiou F, Temperikidis P, Louka A, Asimakos A, Kougias M, Makris D, Zakynthinos E, Xintara M, Papadonta ME, Koutsothymiou A, Zakynthinos SG, Ischaki E. Mentzelopoulos SD, et al. Resusc Plus. 2022 May 26;10:100252. doi: 10.1016/j.resplu.2022.100252. eCollection 2022 Jun. Resusc Plus. 2022. PMID: 35652112 Free PMC article.
References
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, Muchamuel T, Bennett MK, Driessen C, Ball AJ, Kirk CJ. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res 17: 2734–2743, 2011 - PubMed
- Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, Tanaka K, Kirino T. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab 22: 705–710, 2002 - PubMed
- Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, Asano Y, Takahama H, Sasaki H, Higo S, Asakura M, Takashima S, Hori M, Kitakaze M. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 46: 452–462, 2009 - PubMed
- Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J Cardiovasc Pharmacol Ther 16: 239–243, 2011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous