The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein - PubMed (original) (raw)
The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein
Xin Xiong et al. PLoS Biol. 2012.
Abstract
Axonal degeneration is a hallmark of many neuropathies, neurodegenerative diseases, and injuries. Here, using a Drosophila injury model, we have identified a highly conserved E3 ubiquitin ligase, Highwire (Hiw), as an important regulator of axonal and synaptic degeneration. Mutations in hiw strongly inhibit Wallerian degeneration in multiple neuron types and developmental stages. This new phenotype is mediated by a new downstream target of Hiw: the NAD+ biosynthetic enzyme nicotinamide mononucleotide adenyltransferase (Nmnat), which acts in parallel to a previously known target of Hiw, the Wallenda dileucine zipper kinase (Wnd/DLK) MAPKKK. Hiw promotes a rapid disappearance of Nmnat protein in the distal stump after injury. An increased level of Nmnat protein in hiw mutants is both required and sufficient to inhibit degeneration. Ectopically expressed mouse Nmnat2 is also subject to regulation by Hiw in distal axons and synapses. These findings implicate an important role for endogenous Nmnat and its regulation, via a conserved mechanism, in the initiation of axonal degeneration. Through independent regulation of Wnd/DLK, whose function is required for proximal axons to regenerate, Hiw plays a central role in coordinating both regenerative and degenerative responses to axonal injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
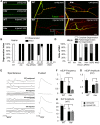
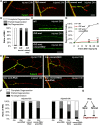
Figure 1. Mutations in hiw strongly delay Wallerian degeneration of motoneuron axons and synapses after injury.
(A) In Drosophila third instar larvae, single axons are labeled by expression of UAS-mCD8::GFP with the m12-Gal4 driver. In a wild-type (WT) background, axons distal to the injury site have completely degenerated within 24 h after nerve crush injury, however axons remain intact in the hiw Δ_N_ mutant background. (B) Quantification of axon degeneration index in different genotypes. (See Materials and Methods and for quantification methods). The degeneration index (percent degenerated) is shown with white bars, while black bars show the complementary percentage (percent intact). The genotypes are: (UAS-mCD8::GFP/+; m12-Gal4/+), (hiw Δ_N_ ;UAS-mCD8::GFP/+; m12-Gal4/+), (UAS-mCD8::GFP/+; m12-Gal4/UAS-hiw-ΔRING), (UAS-mCD8::GFP/UAS-UBP2; m12-Gal4/+), (UAS-mCD8::GFP/+; m12-Gal4/UAS-WldS). (C) Representative images of muscle 4 NMJs in wild-type (WT) or hiw Δ_N_ mutants 24 h after injury. In WT animals, the presynaptic marker Futsch (green) completely disappears, while the neuronal membrane, labeled with antibodies to HRP (red), remain in discontinuous fragments. In contrast, NMJs in the hiw mutant (which are overgrown in an uninjured animal [8]) remain continuous and intact after injury. Of note, Futsch staining does not completely cover some synaptic branches in hiw mutant, but quantification of the extent of Futsch coverage (as in [20]) shows no significant difference between injured and uninjured hiw mutants (unpublished data). (D) Quantification of NMJ degeneration. White bars represent percentage of NMJs that have completely degenerated, defined by a complete loss of Futsch staining from the NMJ. Gray bars represent the percentage of NMJs that are partially degenerated, defined by a partial fragmentation Futsch staining and neuronal membrane (see Materials and Methods). Black bars represent the percentage of NMJs that are intact. The genotypes are: (Canton S), (hiw Δ_N_ ), (hiwND8, BG380-Gal4; UAS-hiw/+), (BG380-Gal4;; UAS-hiw-ΔRING/+), (BG380-Gal4; UAS-UBP2/+), (BG380-Gal4;; UAS-WldS/+). (E) Representative traces of evoked and spontaneous neurotransmitter release recorded from wild-type (Canton S) and hiw mutant (hiw Δ_N_ ) larvae before or 24 h after injury. Calibration: 200 ms, 2 mV for spontaneous release; 30 ms, 5 mV for evoked release. (F–H) Histograms showing (F) average spontaneous miniature EJP frequency, (G) spontaneous miniature EJP amplitude, and (H) evoked EJP amplitude, either from uninjured (black bars) or injured (24 h after injury, gray bars), in Canton S (WT) or hiw Δ_N_ larvae. n = 10 recordings for each genotype. In WT injured larvae, only one single miniature event (amplitude 2 mV) was observed in all ten recordings. Of note, in uninjured larvae the amplitudes of evoked and miniature EJPs were smaller in hiw mutant, as previously reported . Scale bars = 12.5 µm, error bars represent standard error; ***p<0.001; ns, not significant, _p_>0.05 in _t_-test.
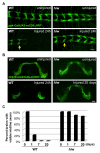
Figure 2. Wallerian degeneration in neurons of different neural types and developmental stages is strongly arrested in hiw mutants.
(A) The nerve terminals of class IV sensory neurons in the ventral nerve cord, visualized by driving UAS-mCD8::RFP with ppk-Gal4, are completely degenerated and cleared within 24 h after injury in wild-type (WT) animals, however these injured axons remain intact in the hiw Δ_N_ mutant. (Because the site of injury was in segment A2, all axons whose terminals are to the right of the yellow arrows have been injured). (B) Olfactory neuron axons in adult flies are labeled by driving expression of UAS-mCD8::GFP with OR47b-Gal4. These axons degenerate within 1 d after antenna removal in wild-type flies, however in hiwΔ_N_ mutants these axons remain intact even 20 d after axotomy. (C) Quantification of the percentage of animals which retain GFP-labeled commissural axons (scored as described in [2],[35]), in a time course after axotomy. Scale bars = 12.5 µm.
Figure 3. Role of the Wnd/DLK MAPKKK in Hiw-regulated degeneration.
(A) OK6-Gal4, UAS-mCD8-GFP labeled motoneuron axons (green) are severely fragmented in wild-type (WT) axons 24 h after injury, while they remain intact in hiw Δ_N_ mutants (hiw Δ_N_ ; OK6-Gal4/UAS-mCD8::GFP). Axons in hiw; wnd double mutants (hiw Δ_N_ ; OK6-Gal4/UAS-mCD8-GFP; wnd1/wnd2) are only mildly fragmented 24 h after injury, implying that mutation of wnd only partially suppressed the hiw mutant degeneration phenotype. (B) Representative muscle 4 NMJs labeled by immunostaining for Futsch (green) and HRP (neuronal membrane, red) in hiw Δ_N_ mutants or hiw; wnd double mutants (hiw Δ_N_ ;;wnd1/wnd2). At 24 h after injury, NMJs have completely degenerated in wild-type (Figure 1) but are intact in hiw mutants. In hiw;wnd double mutants, some NMJs have completely degenerated (upper panel), while others remain intact (lower panel). (C) Quantification of the percentage of NMJs that are completely degenerated, partially degenerated, or intact (see Materials and Methods) for the following genotypes: (Canton S), (hiw Δ_N_ ), (hiw Δ_N_ ;;wnd1/wnd2), (BG380-Gal4; UAS-wnd/+). Scale bars = 12.5 µm.
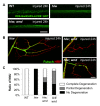
Figure 4. Regulation of Wallerian degeneration by Hiw depends upon endogenous Nmnat
(A) Nmnat is required for the protective phenotype of hiw. m12-Gal4, UAS-mCD8::GFP labeled axons (green) 24 h after injury in animals either mutant for hiw (hiwND8,UAS-Dcr2; UAS-mCD8::GFP/+; m12-Gal4/+) or mutant for in hiw mutant and depleted for nmnat by RNA interference (hiwND8, UAS-Dcr2; UAS-mCD8::GFP/UAS-nmnat-RNAi; m12-Gal4/+). (B) Degeneration index for the m12-Gal4, UAS-mCD8::GFP labeled single axons at different time points after injury in the following genotypes: (UAS-Dcr2; UAS-mCD8::GFP/+; m12-Gal4/+), (UAS-Dcr2; UAS-mCD8-GFP/UAS-nmnat-RNAi; m12-Gal4/+), (hiwND8, UAS-Dcr2; UAS-mCD8::GFP/+; m12-Gal4/+), (hiwND8, UAS-Dcr2; UAS-mCD8::GFP/UAS-nmnat-RNAi; m12-Gal4/+), (hiwND8, BG380-Gal4; UAS-nmnat/UAS-nmnat-RNAi; m12-Gal4,UAS-mCD8::GFP/+). RNAi depletion of nmnat alone only modestly affects the rate of degeneration (compare blue to black); however, it strongly inhibits the protection observed in the hiw mutant (compare orange to red). (C) Representative images of NMJs at muscle 4 24 h after injury in hiw mutants (hiwND8, BG380-Gal4; UAS-Dcr2/+) or hiw mutants depleted for nmnat in neurons (hiwND8, BG380-Gal4; UAS-Dcr2/UAS-nmnat-RNAi). Futsch staining in green labels cytoskeleton structure and HRP staining in red labels neuronal membrane. (D) Quantification of the percentage of NMJs that are completely degenerated, partially degenerated or intact in the above genotypes. (E) Quantification of average bouton numbers per NMJ at muscle 4 in the following genotypes: (BG380-Gal4, UAS-Dcr2), (BG380-Gal4, UAS-Dcr2; UAS-nmnat-RNAi/+), (hiwND8,BG380-Gal4; UAS-Dcr2/+), (hiwND8, BG380-Gal4; UAS-Dcr2/UAS-nmnat-RNAi). Scale bars = 12.5 µm, error bars represent standard error; *p<0.05; **_p_<0.01; ***_p_<0.001; ns, not significant, _p_>0.05 in _t_-test.
Figure 5. Wnd/DLK and Nmnat protect axons through parallel mechanisms downstream of Hiw.
(A) Representative muscle 4 NMJs at 24 h after injury immunostained for Futsch (green) and HRP (neuronal membrane, red) for the following genotypes: WT (Canton S), neuronally overexpressed nmnat (BG380-Gal4; UAS-HA-nmnat/+), or overexpressed nmnat in a wnd mutant background (BG380-Gal4; UAS-HA-nmnat/+; wnd1/wnd2). Overexpression of nmnat protected NMJs from degeneration and this protection was not compromised by mutations in wnd. (B) Quantification of NMJ degeneration in the above genotypes. (C) UAS-mCD8::GFP/+; m12-Gal4/+ labeled singles axons (green) 24 h after injury in WT (UAS-Dcr2; UAS-mCD8::GFP/+; m12-Gal4/+), when overexpressing wnd (UAS-Dcr2; UAS-mCD8-GFP/+; m12-Gal4/UAS-wnd), or when overexpressing wnd in conjunction with nmnat RNAi (UAS-Dcr2; UAS-mCD8-GFP/UAS-nmnat-RNAi; m12-Gal4/UAS-wnd). Reducing Nmnat levels by this method had no effect upon the protection caused by overexpression of wnd. (D) Degeneration index of the m12-Gal4, UAS-mCD8::GFP labeled single axons at different time points after injury in the above genotypes. (E) Representative images of NMJs at muscle 4 18 h after injury stained for Futsch (green) and HRP (red) in the following genotypes: (hiwND8,BG380-Gal4; UAS-Dcr2/+), (hiwND8, BG380-Gal4;UAS-Dcr2/+;UAS-wnd-RNAi/+), (hiwND8, BG380-Gal4; UAS-Dcr2/UAS-nmnat-RNAi), (hiwND8, BG380-Gal4; UAS-Dcr2/UAS-nmnat-RNAi; UAS-wnd-RNAi/+). (F) Quantification of the percentage of NMJs that are completely degenerated, partially degenerated, or intact at 12 h or 18 h after injury, for the genotypes described above. (G) Model: Wnd and Nmnat inhibit axonal degeneration through independent pathways downstream of Hiw. Scale bars = 12. 5 µm, error bars represent standard error; *p<0.05; ns, not significant, _p_>0.05 in _t_-test.
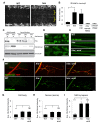
Figure 6. Hiw negatively regulates the levels of Nmnat protein in axons and synapses.
(A) Hiw regulates endogenous Nmnat protein in neuropil. In hiw Δ_N_ mutants, Nmnat protein can be detected within the neuropil of the ventral nerve cord, denoted with asterisks. This area of the nerve cord is devoid of cell bodies and enriched in neurites and synapses. (B) Quantification (relative levels) of Nmnat staining in neuropil, for WT (w118), hiw mutant (hiw Δ_N_), hiw,Ctrl (hiw Δ_N_, BG380-Gal4), hiw, nmnat-RNAi (hiw Δ_N_, BG380-Gal4, UAS-nmnat-RNAi), and nmnat-RNAi (BG380-Gal4, UAS-nmnat-RNAi). See Materials and Methods. (C) Western blot with adult heads or larval brains to compare total protein levels of HA-Nmnat in wild-type (ctrl) and hiw mutant backgrounds. The UAS-HA::nmnat transgene is expressed in neurons with the BG380-Gal4 driver, and males are used for all experiments. (D–F) The UAS-HA-Nmnat transgene was expressed in motoneurons with the OK6-Gal4 driver, in wild-type (OK6-Gal4/UAS-HA::nmnat), hiw mutant (hiw Δ_N_;OK6-Gal4/UAS-HA::nmnat) and hiw; wnd double mutant (hiw Δ_N_ ;OK6-Gal4/UAS-HA::nmnat;wnd1/wnd 2 ) backgrounds. HA-Nmnat protein is detected by immunostaining for HA. (D) Representative images of HA-Nmnat in motoneuron cell bodies, (E) segmental (peripheral) nerves, and (F) NMJ synapses, stained for anti-HA (green) and HRP (neuronal membrane, red). (G–I) Quantification of the average HA-Nmnat intensity for the above genotypes in (G) cell bodies, (H) segmental nerves, and (I) NMJ synapses at muscle 4. See Materials and Methods for details about quantification methods. In hiw mutants, Nmnat intensity is increased, particularly at NMJ synapses. Loss of wnd, in hiw;wnd double mutants, has no effect upon this increase. Scale bars = 12.5 µm, error bars represent standard error; *p<0.05; ***_p_<0.001; ns, not significant, _p_>0.05 in _t_-test.
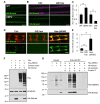
Figure 7. Hiw and ubiquitination down-regulate Nmnat protein.
(A) The UAS-HA-nmnat transgene is expressed in motoneurons with OK6-Gal4 driver in a wild-type (WT)_ genetic background (OK6-Gal4,UAS-HA::nmnat/+) or when the yeast deubiquitinase UBP2 is co-expressed (OK6-Gal4,UAS-HA::nmnat/UAS_-UBP2_). Segmental nerves stained with anti-HA antibody (green). (B) Transgenic HA-nmnat is co-expressed with a control _UAS_- construct (BG380-Gal4; UAS-HA::nmnat/+; UAS-nls::GFP/+) or with the full-length hiw cDNA (BG380-Gal4; UAS-HA::nmnat/UAS-hiw). Segmental nerves stained for HA (green) and HRP (neuronal membrane, violet). (C) Quantification of the average HA-Nmnat intensity in segmental nerves in (A) and (B). The average intensity is normalized to the control for each experimental group. (D) Distal axons and axon terminals of ppk-Gal4,UAS-mCD8::RFP labeled sensory neurons stained for HA (green) and RFP (red). The UAS-HA-Nmnat transgene is co-expressed with a control _UAS_- construct (UAS-HA::nmnat/+; ppk-Gal4,UAS-mCD8::RFP/UAS-nls::GFP), or the full-length hiw cDNA (UAS-HA::nmnat/UAS-hiw; ppk-Gal4,UAS-mCD8::RFP/+), or a dominant negative hiw transgene mutated for conserved cysteines in the RING domain _(UAS-HA::nmnat/+; ppk-Gal4,UAS-mCD8::RFP/UAS-hiw-Δ_RING). (E) Quantification of the average HA-Nmnat intensity in the sensory neuron axon terminals for the above genotypes. See Materials and Methods for the quantification method. (F) Hiw can down-regulate Nmnat protein in S2R+ cells. S2R+ cells were co-transfected with pUAST-HA::Nmnat, and either pUAST-GFP (control vector), pUAST-Hiw, or Hiw-ΔRING. All cells were co-transfected with pMT-Gal4 and induced with 0.5 mM copper sulfate for 24 h. Hiw is not expressed endogenously in S2R+ cells, and numerous breakdown products are observed for the ectopically expressed Hiw protein. The reduction in ectopic HA-Nmnat levels in lane 3 indicates that Hiw is capable of post-transcriptionally regulating Nmnat, and that the RING domain is required for this activity (lane 4). (G) Nmnat and Hiw-ΔRING form a physical interaction. Co-immunoprecipitation assays were performed from S2R+ cells lysate either co-transfected with pUAST-HA::Nmnat and pUAST-Hiw-ΔRING (lane 5) or mixed lysates from individual pUAST-HA::Nmnat and pUAST-Hiw-ΔRING transfections (lane 6). HA-Nmnat was immunoprecipitated by mouse anti-HA antibody against the HA tag on Nmnat. Despite the fact that Hiw-ΔRING was significantly degraded in S2R+ cell lysate (detected by Western blotting for Hiw antibody), a significant portion of Hiw-ΔRING protein co-immunoprecipitated with HA-Nmnat. The Input lanes (1–3) represent 1/25 of the total extract used for each immunoprecipitation. Scale bars = 12.5 µm; error bars represent standard error; ***p<0.001 in _t_-test.
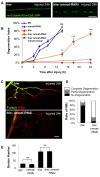
Figure 8. Hiw promotes Nmnat protein turnover in the injured distal axons and synapses
(A) Distal axons and synapses of ppk-Gal4,UAS-mCD8::RFP labeled sensory neurons, located in the ventral nerve cord, either before or 24 h after injury. Transgenic HA::nmnat is expressed in a wild-type (WT) genetic background (UAS-HA::nmnat/+; ppk-Gal4,UAS-mCD8::RFP/+), hiw Δ_N_ mutant (hiw Δ_N_ ; UAS-HA::nmnat/+; ppk-Gal4,UAS-mCD8::RFP/+) or co-expressed with UBP2 (UAS-HA::nmnat/UAS-UBP2; ppk-Gal4,UAS-mCD8::RFP/+). Consistent with observations in motoneurons (Figure 6), mutation of hiw or co-expression of UBP2 causes a dramatic elevation in HA-Nmnat protein, and this does not disappear after injury, in dramatic contrast to the disappearance of HA-Nmnat in wild-type (WT) animals. Of note, the nerve terminals of the ppk-Gal4 labeled axons appear to be overgrown in hiw and UBP2 expressing mutants, similar to previous descriptions in other neuron types ,. Hence the quantification shown in (B) is normalized to the size of the nerve terminals (labeled by mCD8-RFP). Due to the expression of HA-nmnat, no axons are degenerating in any of the above genotypes. (B) Quantification of the average HA-Nmnat intensity in ppk-Gal4 expressing sensory neuron axon terminals, within the most posterior four segments of the ventral nerve cord. Error bars represent standard error. ns, not significant, _p_>0.05. (C) Model: Hiw promotes multiple independent responses to injury, through independent pathways. Hiw promotes degeneration in the distal stump by down-regulating Nmnat, and concurrently regulates regeneration (and protection from degeneration [15],[30]) in the proximal stump by regulating Wnd. Since Hiw may localize and function in distal axons, injury may relieve the inhibition of Wnd by Hiw in the proximal stump. Scale bars = 12.5 µm, error bars represent standard error; ***p<0.001; ns, not significant, _p_>0.05 in _t_-test.
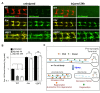
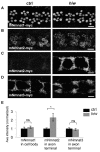
Figure 9. Hiw is capable of regulating mouse Nmnat2, but not mouse Nmnat1 or Nmnat3, protein.
(A, B) UAS-mNmnat1::myc and UAS-mNmnat2::myc transgenes, expressed in motoneurons with the BG380-Gal4 driver, show no difference in cell body levels between control and hiwND8 mutants. (C–D) UAS-mNmnat2::myc, and UAS-mNmnat3::myc were expressed with ppk-Gal4 in order to visualize localization at sensory neuron axon terminal in the nerve cord. The levels of mNmnat2-myc protein at axon terminals were increased in hiwND8 mutants. In contrast, mNmant3-myc protein levels were similar in between control and hiwND8 mutant genotypes. (E) Quantification of average intensity of mNmnat1 in cell body, and mNmnat2 and mNmnat3 in axon terminals from (A–D). Scale bars = 12.5 µm, error bars represent standard error; *p<0.05; ns, not significant, _p_>0.05 in _t_-test.
Similar articles
- SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury.
Brace EJ, Wu C, Valakh V, DiAntonio A. Brace EJ, et al. J Neurosci. 2014 Jun 18;34(25):8398-410. doi: 10.1523/JNEUROSCI.4715-13.2014. J Neurosci. 2014. PMID: 24948796 Free PMC article. - An Atypical SCF-like Ubiquitin Ligase Complex Promotes Wallerian Degeneration through Regulation of Axonal Nmnat2.
Yamagishi Y, Tessier-Lavigne M. Yamagishi Y, et al. Cell Rep. 2016 Oct 11;17(3):774-782. doi: 10.1016/j.celrep.2016.09.043. Cell Rep. 2016. PMID: 27732853 Free PMC article. - A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade.
Xiong X, Collins CA. Xiong X, et al. J Neurosci. 2012 Jan 11;32(2):610-5. doi: 10.1523/JNEUROSCI.3586-11.2012. J Neurosci. 2012. PMID: 22238096 Free PMC article. - Wallerian degeneration, wld(s), and nmnat.
Coleman MP, Freeman MR. Coleman MP, et al. Annu Rev Neurosci. 2010;33:245-67. doi: 10.1146/annurev-neuro-060909-153248. Annu Rev Neurosci. 2010. PMID: 20345246 Free PMC article. Review. - Why is NMNAT Protective against Neuronal Cell Death and Axon Degeneration, but Inhibitory of Axon Regeneration?
Tang BL. Tang BL. Cells. 2019 Mar 21;8(3):267. doi: 10.3390/cells8030267. Cells. 2019. PMID: 30901919 Free PMC article. Review.
Cited by
- Local translatome sustains synaptic function in impaired Wallerian degeneration.
Paglione M, Restivo L, Zakhia S, Llobet Rosell A, Terenzio M, Neukomm LJ. Paglione M, et al. EMBO Rep. 2024 Oct 31. doi: 10.1038/s44319-024-00301-8. Online ahead of print. EMBO Rep. 2024. PMID: 39482489 - Rab11 suppresses neuronal stress signaling by localizing dual leucine zipper kinase to axon terminals for protein turnover.
Kim SM, Quagraine Y, Singh M, Kim JH. Kim SM, et al. Elife. 2024 Oct 30;13:RP96592. doi: 10.7554/eLife.96592. Elife. 2024. PMID: 39475475 Free PMC article. - Pathogenic variants in TMEM184B cause a neurodevelopmental syndrome via alteration of metabolic signaling.
Chapman KA, Ullah F, Yahiku ZA, Kodiparthi SV, Kellaris G, Correia SP, Stödberg T, Sofokleous C, Marinakis NM, Fryssira H, Tsoutsou E, Traeger-Synodinos J, Accogli A, Salpietro V, Striano P, Berger SI, Pond KW, Sirimulla S, Davis EE, Bhattacharya MR. Chapman KA, et al. medRxiv [Preprint]. 2024 Jul 1:2024.06.27.24309417. doi: 10.1101/2024.06.27.24309417. medRxiv. 2024. PMID: 39006436 Free PMC article. Preprint. - Ubiquitin ligase and signalling hub MYCBP2 is required for efficient EPHB2 tyrosine kinase receptor function.
Chang C, Banerjee SL, Park SS, Zhang XL, Cotnoir-White D, Opperman KJ, Desbois M, Grill B, Kania A. Chang C, et al. Elife. 2024 Jan 30;12:RP89176. doi: 10.7554/eLife.89176. Elife. 2024. PMID: 38289221 Free PMC article. - Opposing roles of Fos, Raw, and SARM1 in the regulation of axonal degeneration and synaptic structure.
Waller TJ, Collins CA. Waller TJ, et al. Front Cell Neurosci. 2023 Nov 30;17:1283995. doi: 10.3389/fncel.2023.1283995. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38099151 Free PMC article.
References
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, et al. (2006) Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50: 883–895. - PubMed
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, et al. (2003) Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39: 217–225. - PubMed
- D'Souza J, Hendricks M, Le Guyader S, Subburaju S, Grunewald B, et al. (2005) Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc). Development 132: 247–256. - PubMed
- Schaefer AM, Hadwiger GD, Nonet ML (2000) rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26: 345–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS070962/NS/NINDS NIH HHS/United States
- NS07092/NS/NINDS NIH HHS/United States
- NS069844/NS/NINDS NIH HHS/United States
- R56 NS069844/NS/NINDS NIH HHS/United States
- R01 NS069844/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous