Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing - PubMed (original) (raw)
Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing
Simone M T Camps et al. PLoS One. 2012.
Abstract
Azole compounds are the primary therapy for patients with diseases caused by Aspergillus fumigatus. However, prolonged treatment may cause resistance to develop, which is associated with treatment failure. The azole target cyp51A is a hotspot for mutations that confer phenotypic resistance, but in an increasing number of resistant isolates the underlying mechanism remains unknown. Here, we report the discovery of a novel resistance mechanism, caused by a mutation in the CCAAT-binding transcription factor complex subunit HapE. From one patient, four A. fumigatus isolates were serially collected. The last two isolates developed an azole resistant phenotype during prolonged azole therapy. Because the resistant isolates contained a wild type cyp51A gene and the isolates were isogenic, the complete genomes of the last susceptible isolate and the first resistant isolate (taken 17 weeks apart) were sequenced using Illumina technology to identify the resistance conferring mutation. By comparing the genome sequences to each other as well as to two A. fumigatus reference genomes, several potential non-synonymous mutations in protein-coding regions were identified, six of which could be confirmed by PCR and Sanger sequencing. Subsequent sexual crossing experiments showed that resistant progeny always contained a P88L substitution in HapE, while the presence of the other five mutations did not correlate with resistance in the progeny. Cloning the mutated hapE gene into the azole susceptible akuB(KU80) strain showed that the HapE P88L mutation by itself could confer the resistant phenotype. This is the first time that whole genome sequencing and sexual crossing strategies have been used to find the genetic basis of a trait of interest in A. fumigatus. The discovery may help understand alternate pathways for azole resistance in A. fumigatus with implications for the molecular diagnosis of resistance and drug discovery.
Conflict of interest statement
Competing Interests: Paul E. Verweij received research grants from the commercial companies Basilea, BioRad, Gilead, Merck, Pfizer and Schering-Plough, which is a potential competing interest. Author Bas E. Dutilh is a PLOS ONE Editorial Board member, but this does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
Figure 1. Relapsing infection with A. fumigatus in a CGD patient.
During the course of the disease, four A. fumigatus isolates were obtained. Sampling dates of isolates 1 through 4 are indicated. S indicates susceptibility to azoles and R indicates resistance. Minimum inhibitory concentrations (MICs) for itraconazole, voriconazole, and posaconazole are also shown. The patient was treated with voriconazole monotherapy (week −67 to +12), caspofungin+voriconazole combination therapy (week +12 to +90) and caspofungin+posaconazole combination therapy (week +90 to +134). Plasma concentrations at week 86 (voriconazole) and weeks 99, 105, and 123 (posaconazole) are also indicated. The patient died from the pulmonary infection at week 134 .
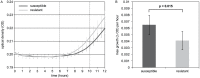
Figure 2. In vitro growth curves of susceptible and resistant progeny.
A. In vitro growth curves of susceptible (n = 12) and resistant (n = 4) progeny. B. The maximum growth per hour during the exponential phase of the growth curve (6–10 hours, between dashed lines of Figure 2A) was averaged (±standard deviation) for susceptible and resistant progeny. The difference is significant according to a Student's T-test (one-tailed, heteroscedastic).
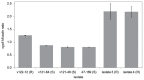
Figure 3. Cyp51A mRNA levels in the parental isolates (47–169, isolate 3, isolate 4) and three of the progeny (v122-12, v121-64, v121-48).
Isolate v122-12 contains only the HapE substitution and not the other five gene mutations. As a control, we selected two other progeny: v121-64 containing only the erg25 mutation and v121-48 containing the erg6/erg25 double mutation. S: susceptible, R: resistant.
Similar articles
- Non-cyp51A Azole-Resistant Aspergillus fumigatus Isolates with Mutation in HMG-CoA Reductase.
Hagiwara D, Arai T, Takahashi H, Kusuya Y, Watanabe A, Kamei K. Hagiwara D, et al. Emerg Infect Dis. 2018 Oct;24(10):1889-1897. doi: 10.3201/eid2410.180730. Emerg Infect Dis. 2018. PMID: 30226177 Free PMC article. - In Vitro Activity of ASP2397 against Aspergillus Isolates with or without Acquired Azole Resistance Mechanisms.
Arendrup MC, Jensen RH, Cuenca-Estrella M. Arendrup MC, et al. Antimicrob Agents Chemother. 2015 Nov 9;60(1):532-6. doi: 10.1128/AAC.02336-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26552973 Free PMC article. - Structural basis of HapEP88L-linked antifungal triazole resistance in Aspergillus fumigatus.
Hortschansky P, Misslinger M, Mörl J, Gsaller F, Bromley MJ, Brakhage AA, Groll M, Haas H, Huber EM. Hortschansky P, et al. Life Sci Alliance. 2020 May 28;3(7):e202000729. doi: 10.26508/lsa.202000729. Print 2020 Jul. Life Sci Alliance. 2020. PMID: 32467317 Free PMC article. - Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature.
Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. Camps SM, et al. Antimicrob Agents Chemother. 2012 Jan;56(1):10-6. doi: 10.1128/AAC.05088-11. Epub 2011 Oct 17. Antimicrob Agents Chemother. 2012. PMID: 22005994 Free PMC article. Review. - Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms.
Chowdhary A, Sharma C, Hagen F, Meis JF. Chowdhary A, et al. Future Microbiol. 2014;9(5):697-711. doi: 10.2217/fmb.14.27. Future Microbiol. 2014. PMID: 24957095 Review.
Cited by
- Sterol Biosynthesis and Azole Tolerance Is Governed by the Opposing Actions of SrbA and the CCAAT Binding Complex.
Gsaller F, Hortschansky P, Furukawa T, Carr PD, Rash B, Capilla J, Müller C, Bracher F, Bowyer P, Haas H, Brakhage AA, Bromley MJ. Gsaller F, et al. PLoS Pathog. 2016 Jul 20;12(7):e1005775. doi: 10.1371/journal.ppat.1005775. eCollection 2016 Jul. PLoS Pathog. 2016. PMID: 27438727 Free PMC article. - The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens.
Arastehfar A, Lass-Flörl C, Garcia-Rubio R, Daneshnia F, Ilkit M, Boekhout T, Gabaldon T, Perlin DS. Arastehfar A, et al. J Fungi (Basel). 2020 Aug 18;6(3):138. doi: 10.3390/jof6030138. J Fungi (Basel). 2020. PMID: 32824785 Free PMC article. Review. - Raw genome sequence data for 13 isogenic Aspergillus fumigatus strains isolated over a 2 year period from a patient with chronic granulomatous disease.
Ballard E, Zoll J, Melchers WJG, Brown AJP, Warris A, Verweij PE. Ballard E, et al. Data Brief. 2019 May 23;25:104021. doi: 10.1016/j.dib.2019.104021. eCollection 2019 Aug. Data Brief. 2019. PMID: 31194082 Free PMC article. - Lysine acetylation as drug target in fungi: an underexplored potential in Aspergillus spp.
Wassano NS, Leite AB, Reichert-Lima F, Schreiber AZ, Moretti NS, Damasio A. Wassano NS, et al. Braz J Microbiol. 2020 Jun;51(2):673-683. doi: 10.1007/s42770-020-00253-w. Epub 2020 Mar 13. Braz J Microbiol. 2020. PMID: 32170592 Free PMC article. Review. - Pulmonary Aspergillosis in Humboldt Penguins-Susceptibility Patterns and Molecular Epidemiology of Clinical and Environmental Aspergillus fumigatus Isolates from a Belgian Zoo, 2017-2022.
Debergh H, Becker P, Vercammen F, Lagrou K, Haesendonck R, Saegerman C, Packeu A. Debergh H, et al. Antibiotics (Basel). 2023 Mar 15;12(3):584. doi: 10.3390/antibiotics12030584. Antibiotics (Basel). 2023. PMID: 36978451 Free PMC article.
References
- Denning DW, Riniotis K, Dobrashian R, Sambatakou H (2003) Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 37 Suppl 3: S265–280. - PubMed
- Howard SJ, Pasqualotto AC, Denning DW (2010) Azole resistance in allergic bronchopulmonary aspergillosis and Aspergillus bronchitis. Clin Microbiol Infect 16: 683–688. - PubMed
- Snelders E, Melchers WJ, Verweij PE (2011) Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol 6: 335–347. - PubMed
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, et al. (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347: 408–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
BED is supported by the Dutch Science foundation (NWO) Veni grant (016.111.075). PEV received research grants from Basilea, BioRad, Gilead, Merck, Pfizer, and Schering-Plough. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials