WIKI4, a novel inhibitor of tankyrase and Wnt/ß-catenin signaling - PubMed (original) (raw)
WIKI4, a novel inhibitor of tankyrase and Wnt/ß-catenin signaling
Richard G James et al. PLoS One. 2012.
Abstract
The Wnt/ß-catenin signaling pathway controls important cellular events during development and often contributes to disease when dysregulated. Using high throughput screening we have identified a new small molecule inhibitor of Wnt/ß-catenin signaling, WIKI4. WIKI4 inhibits expression of ß-catenin target genes and cellular responses to Wnt/ß-catenin signaling in cancer cell lines as well as in human embryonic stem cells. Furthermore, we demonstrate that WIKI4 mediates its effects on Wnt/ß-catenin signaling by inhibiting the enzymatic activity of TNKS2, a regulator of AXIN ubiquitylation and degradation. While TNKS has previously been shown to be the target of small molecule inhibitors of Wnt/ß-catenin signaling, WIKI4 is structurally distinct from previously identified TNKS inhibitors.
Conflict of interest statement
Competing Interests: The authors have read the journal’s policy and have the following conflicts: RTM is a co-founder of, and consultant with, FATE THERAPEUTICS, San Diego.
Figures
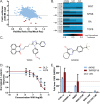
Figure 1. WIKI4 is identified as a novel small molecule inhibitor of the Wnt/ß-catenin pathway.
(A) Scatter plot of a small molecule screen in human A375 melanoma cells stably expressing the ß-catenin Activated Reporter (BAR) driving firefly luciferase with each dot representing a single compound. The red dots represent compounds that exhibited decreased luciferase signal (> two standard deviations below the sample mean), and unchanged cell viability as measured by resazurin. (B) A heat map showing the effects of five Wnt/ß-catenin inhibitors on reporters for the Wnt/ß-catenin, Nuclear Factor Kappa B (NF-kB), Retinoic Acid (RA), and Transforming Growth Factor ß (TGFB) pathways. WIKI4 (arrow) is the only compound that specifically inhibits Wnt/ß-catenin signaling. (C) Chemical structure of WIKI4 (left) and XAV-939 (right). (D) Dose response curves showing that WIKI4 inhibits ß-catenin reporter activity in DLD1 colorectal carcinoma cells and Wnt-stimulated A375 melanoma cells. (E) Inhibition of the expression of the ß-catenin target genes AXIN2 and TNFRSF19 by WIKI4 as assessed by quantitative PCR. DLD cells were transfected with CTNNB1 siRNA as a control 72 hours prior to harvesting for RNA; cells were treated with compounds or DMSO for 16 hours prior to harvesting. The experiments in (D) and (E) are representative of three independent experiments and the error bars represent standard deviation from four technical replicates.
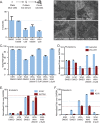
Figure 2. WIKI4 inhibits the functional outcomes of Wnt/ß-catenin signaling.
(A) WIKI4 inhibits colony formation of DLD1 colorectal cancer cells. DLD1 cells were plated individually in 0.5% serum containing medium, and treated with the indicated concentrations of WIKI4 and XAV-939. This experiment is representative of three independent experiments and the error bars represent standard deviation of three technical replicates. (B-F) WIKI4 prevents Wnt3A-dependent differentiation of H1 human embryonic stem cells (hESCs). (B) Culturing hESCs for six days with Wnt3A causes marked morphological changes that are rescued by treatment with WIKI4. Scalebar = 500 µm. (C) Treatment with WIKI4 prevents the decrease in co-expression of markers of undifferentiated hESCs following Wnt3A stimulus. hESCs were stimulated with the indicated treatments and expression of GCTM2 and CD9 was assessed by flow cytometry following six days of treatment. (D-F) The effect of WIKI4 treatment on the expression of genes that are altered during Wnt3A-dependent differentiation of hESCs was assessed by qPCR. hESCs were treated for the indicated conditions for six days, and then analyzed by qPCR for markers of undifferentiated stem cells (NANOG, POU5F1) (D), endoderm (SOX17, GATA6) (E), and mesoderm (T, KDR) (F). The data was normalized to 100,000 copies of GAPDH and plotted as a ratio to the untreated hESCs (cultured in KSR media). The data in the experiments presented in B-F are representative of three independent experiments and the error represents standard deviation of technical replicates. In B-F, LCM = control L cell CM, WNT3A = Wnt3a CM; both 50% (vol/vol) in KSR medium.
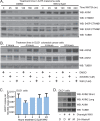
Figure 3. WIKI4 increases the steady-state abundance of the Wnt/ß-catenin inhibitory protein, AXIN1.
(A) WIKI4 prevents degradation of AXIN1 following stimulation with Wnt3A. A375 melanoma cells were stimulated with 10% (vol/vol) Wnt3A CM for the indicated time periods with or without WIKI4 treatment, lysed and analyzed by western blot using the indicated antibodies. (B) WIKI4 increases the steady-state abundance of AXIN1 and AXIN2 protein. DLD1 colorectal carcinoma cells were incubated with DMSO, WIKI4 or XAV-939 for the indicated times, lysed and analyzed by western blot. (C) WIKI4 does not significantly affect the steady-state RNA abundance of AXIN1. DLD1 colorectal carcinoma cells were incubated with WIK4 for the indicated times, and processed for qPCR to assess changes in the steady-state abundance of AXIN1 transcript. This data is representative of two independent experiments and the error bars represent standard deviation. (D) WIKI4-dependent increases in AXIN1 protein abundance can be maintained by treatment with a proteasome inhibitor. DLD1 colorectal carcinoma cells were treated overnight with WIKI4, and after washing were then incubated for two hours with DMSO (D), WIKI4 (W), or the proteasome inhibitor MG132 (M). The cells were lysed and analyzed by western blotting for the indicated antibodies.
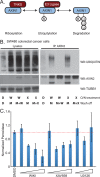
Figure 4. WIKI4 prevents ubiquitylation of AXIN and inhibits the enzymatic activity of TNKS2.
(A) Schematic showing a model of how AXIN proteins are sequentially ADP-Ribosylated and then poly-ubiquitylated prior to their degradation by the proteasome. (B) WIKI4 inhibits ubiquitylation of AXIN2 in SW480 colorectal carcinoma cells. SW480 cells were treated overnight with DMSO (D), 2.5 µM WIKI4 (W) or 2.5 µM XAV-939 (X). Following a brief wash, the cells were then incubated for two hours with DMSO (D), 10 µM MG132 (M) or MG132 and one of the Wnt/ß-catenin pathway inhibitors. Lysates and AXIN2 immunoprecipitates from this experiment were processed for western blotting with the indicated antibodies. (C) WIKI4 inhibits the enzymatic activity of TNKS2. Recombinant GST-TNKS2 was bound to 96-well plates coated with glutathione. Auto-ADP-ribosylation assays were carried out using biotinylated substrate in the context of the indicated treatments. The amount of TNKS2 auto-ribosylation was quantified by performing chemiluminescent detection of the reaction between streptavidin conjugated to horseradish peroxidase and biotinylated substrate. U0126 was used as a negative control.
Similar articles
- Structural basis and selectivity of tankyrase inhibition by a Wnt signaling inhibitor WIKI4.
Haikarainen T, Venkannagari H, Narwal M, Obaji E, Lee HW, Nkizinkiko Y, Lehtiö L. Haikarainen T, et al. PLoS One. 2013 Jun 6;8(6):e65404. doi: 10.1371/journal.pone.0065404. Print 2013. PLoS One. 2013. PMID: 23762361 Free PMC article. - The Discovery and Characterization of K-756, a Novel Wnt/β-Catenin Pathway Inhibitor Targeting Tankyrase.
Okada-Iwasaki R, Takahashi Y, Watanabe Y, Ishida H, Saito J, Nakai R, Asai A. Okada-Iwasaki R, et al. Mol Cancer Ther. 2016 Jul;15(7):1525-34. doi: 10.1158/1535-7163.MCT-15-0938. Epub 2016 Apr 25. Mol Cancer Ther. 2016. PMID: 27196752 - Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling.
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Huang SM, et al. Nature. 2009 Oct 1;461(7264):614-20. doi: 10.1038/nature08356. Epub 2009 Sep 16. Nature. 2009. PMID: 19759537 - Regulation of Wnt/β-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding.
Mariotti L, Pollock K, Guettler S. Mariotti L, et al. Br J Pharmacol. 2017 Dec;174(24):4611-4636. doi: 10.1111/bph.14038. Epub 2017 Nov 5. Br J Pharmacol. 2017. PMID: 28910490 Free PMC article. Review. - Wnt/beta-catenin pathway: modulating anticancer immune response.
Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Pai SG, et al. J Hematol Oncol. 2017 May 5;10(1):101. doi: 10.1186/s13045-017-0471-6. J Hematol Oncol. 2017. PMID: 28476164 Free PMC article. Review.
Cited by
- DGDRP: drug-specific gene selection for drug response prediction via re-ranking through propagating and learning biological network.
Pak M, Bang D, Sung I, Kim S, Lee S. Pak M, et al. Front Genet. 2024 Sep 20;15:1441558. doi: 10.3389/fgene.2024.1441558. eCollection 2024. Front Genet. 2024. PMID: 39371421 Free PMC article. - Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease.
Maiese K. Maiese K. Neural Regen Res. 2015 Apr;10(4):518-28. doi: 10.4103/1673-5374.155427. Neural Regen Res. 2015. PMID: 26170801 Free PMC article. Review. - Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics.
Haseeb M, Pirzada RH, Ain QU, Choi S. Haseeb M, et al. Cells. 2019 Nov 3;8(11):1380. doi: 10.3390/cells8111380. Cells. 2019. PMID: 31684152 Free PMC article. Review. - Tankyrase inhibition interferes with junction remodeling, induces leakiness, and disturbs YAP1/TAZ signaling in the endothelium.
Ma N, Wibowo YC, Wirtz P, Baltus D, Wieland T, Jansen S. Ma N, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1763-1789. doi: 10.1007/s00210-023-02720-1. Epub 2023 Sep 23. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37741944 Free PMC article. - Protein kinase PKN1 represses Wnt/β-catenin signaling in human melanoma cells.
James RG, Bosch KA, Kulikauskas RM, Yang PT, Robin NC, Toroni RA, Biechele TL, Berndt JD, von Haller PD, Eng JK, Wolf-Yadlin A, Chien AJ, Moon RT. James RG, et al. J Biol Chem. 2013 Nov 29;288(48):34658-70. doi: 10.1074/jbc.M113.500314. Epub 2013 Oct 10. J Biol Chem. 2013. PMID: 24114839 Free PMC article.
References
- Tanaka SS, Kojima Y, Yamaguchi YL, Nishinakamura R, Tam PP (2011) Impact of WNT signaling on tissue lineage differentiation in the early mouse embryo. Dev Growth Differ 53: 843–856. - PubMed
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480. - PubMed
- Shimizu N, Kawakami K, Ishitani T (2012) Visualization and exploration of Tcf/Lef function using a highly responsive Wnt/beta-catenin signaling-reporter transgenic zebrafish. Dev Biol. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99/R00 1K99HL103768-01/HL/NHLBI NIH HHS/United States
- R00 HL103768/HL/NHLBI NIH HHS/United States
- U01 HL100395/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K99 HL103768/HL/NHLBI NIH HHS/United States
- T32AR056969/AR/NIAMS NIH HHS/United States
- T32 AR056969/AR/NIAMS NIH HHS/United States
- P01 GM081619/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources