Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma - PubMed (original) (raw)
doi: 10.1371/journal.pone.0051044. Epub 2012 Dec 5.
Ilkka Alahuhta, Sini Nurmenniemi, Juho Suojanen, Riitta Palovuori, Susanna Teppo, Timo Sorsa, Carlos López-Otín, Taina Pihlajaniemi, Tuula Salo, Ritva Heljasvaara, Pia Nyberg
Affiliations
- PMID: 23227231
- PMCID: PMC3515547
- DOI: 10.1371/journal.pone.0051044
Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma
Mari Aikio et al. PLoS One. 2012.
Abstract
The turnover of extracellular matrix liberates various cryptic molecules with novel biological activity. Among these are the collagen-derived anti-angiogenic fragments, some of which are suggested to affect carcinoma cells also directly. Arresten is an endogenous angiogenesis inhibitor that is derived from the non-collagenous domain of the basement membrane collagen IV α1 chain. As the mere prevention of tumor angiogenesis leads to hypoxia that can result in selection of more aggressive cell types and reduces the efficacy of chemotherapy, we aimed here to elucidate how arresten influences the aggressive human carcinoma cells. Arresten efficiently inhibited migration and invasion of HSC-3 tongue carcinoma cells in culture and in an organotypic model. Subcutaneous Arr-HSC xenografts grew markedly more slowly in nude mice and showed reduced tumor cell proliferation, vessel density and local invasiveness. In the organotypic assay, HSC-3 cells overproducing arresten (Arr-HSC) showed induction of cell death. In monolayer culture the Arr-HSC cells grew in aggregated cobblestone-like clusters and, relative to the control cells, showed increased expression and localization of epithelial marker E-cadherin in cell-cell contacts. Application of electric cell-substrate impedance sensing (ECIS) further supported our observations on altered morphology and motility of the Arr-HSC cells. Administration of a function-blocking α1 integrin antibody abolished the impedance difference between the Arr-HSC and control cells suggesting that the effect of arresten on promotion of HSC-3 cell-cell contacts and cell spreading is at least partly mediated by α1β1 integrin. Collectively, our data suggest novel roles for arresten in the regulation of oral squamous carcinoma cell proliferation, survival, motility and invasion through the modulation of cell differentiation state and integrin signaling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Arresten inhibits migration of HSC-3 cells.
A. 30 000 Ctrl-HSC and Arr-HSC cells were allowed to migrate through Transwell inserts and the number of migrated cells was counted under a microscope at 50×magnification. Mann-Whitney U-test, ***p<0.001, (n = total number of fields analyzed, 2–4 fields per Transwell insert). B. 30 000 HSC-3 cells were allowed to migrate through Transwell inserts in the presence of human recombinant purified arresten (5 and 20 µg/ml) and the number of migrated cells was counted as described above. Mann-Whitney U-test, **p<0.01, (n = total number of fields analyzed, 3–5 fields per Transwell insert). C. Scratch wound healing assay with Ctr-HSC and Arr-HSC clones in which the closure of the wound was measured at 0, 16 and 48 h. Scale bar 50 µm**.** E. Quantification of scratch wound healing in the Ctrl-HSC and Arr-HSC clones. Mann-Whitney U-test, ***p<0.001, (n = 70 fields at 0, 16 and 48 h per clone).
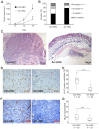
Figure 2. Effects of arresten on HSC-3 xenografts.
A. One million Ctrl-HSC and Arr-HSC cells were injected subcutaneously into the flanks of nude mice and tumor growth was monitored over 16 days. Students t-test, *p<0.05, (n = 10 mice per group). B. Local invasiveness of the tumors. C. Representative hematoxylin-eosin stainings of HSC-3 xenografts. Scale bar 100 µm. D–E. HSC-3 xenografts were stained for the proliferation marker Ki-67 (brown) and the cell proliferation was defined as a percentage of Ki-67-positive cells among the total number of carcinoma cells per microscopic field (400×magnification; n = number of fields analyzed, 3–5 fields per xenograft). Scale bar 50 µm. F–G. The tumor blood vessels were stained with a CD31 antibody and counted under a microscope (200×magnification; n = number of fields analyzed, 3–5 fields per xenograft). Mann-Whitney U-test, ***p<0.001. Scale bar 100 µm.
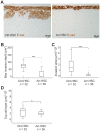
Figure 3. Arresten efficiently inhibits HSC-3 carcinoma cell invasion in an organotypic model.
A. Ctrl-HSC and Arr-HSC cells (7×105) were cultured on top of a collagen gel embedded with human gingival fibroblasts (7×105). The organotypic sections were stained with E-cadherin antibody (brown). Scale bar 20 µm. Tumor cell invasion and growth were quantified by measuring the maximal invasion depth (B), invasion area (C) and area of the top cell layer of pancytokeratin stained sections (D). Mann-Whitney U-test, ***p<0.001, *p<0.05, (n = total number of fields analyzed, 4–5 fields per organotypic section).
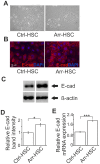
Figure 4. Arresten promotes an epithelial morphology of HSC-3 cells and increases the amount E-cadherin in cell-cell contacts.
A. Arresten overexpression induced a cobblestone-like appearance in HSC-3 tongue squamous cell carcinoma cells (200×magnification). B. Immunostaining of E-cadherin (red) in cultured Ctrl-HSC and Arr-HSC cells (blue, DAPI). Scale bar 10 µm. C. 10 µg of total protein from lysed cell extracts was analyzed by Western blotting with E-cadherin antibody. β-actin was used as a loading control. D. The relative band intensities were quantified (n = 3 Western analyses from separate protein extractions; mean ± SEM). E. mRNA expression of E-cadherin in cultured Ctrl-HSC (N = 6, n = 12) and Arr-HSC (N = 3, n = 6) cells (N = number of clones analyzed; n = number of samples analyzed). The expression levels were normalized to that of the GAPDH housekeeping gene and are presented relative to values obtained for Ctrl-cells (mean ± SEM) Students t-test, ***p<0.001, *p<0.05.
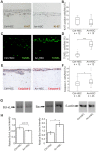
Figure 5. Arresten increases apoptosis of HSC-3 carcinoma cells in the organotypic model.
A. Organotypic sections were stained for the proliferation marker Ki-67 (brown). B. Proliferation was defined as a percentage of Ki-67-positive cells among the total number of carcinoma cells per microscopic field (200×magnification; n = total number of fields analyzed, 3–5 fields per organotypic section). C–F. Apoptotic cells were detected by TUNEL assay (green) and caspase-3 staining (red). Apoptotic cell death was quantified in terms of TUNEL (D) and caspase-3-positive (F) cells as a percentage of total number of carcinoma cells per microscopic field (200×magnification; n = total number of fields analyzed, 3–5 fields per organotypic section). Mann-Whitney U-test, ***p<0.001, *p<0.05. G. 20 µg of total protein of lysed cell extracts was separated by SDS-PAGE and immunoblotted with antibodies against signaling molecules of the Bcl-family apoptosis pathway, anti-apoptotic Bcl-xL and pro-apoptotic Bax. β-actin was used as a loading control. H. The relative band intensities were quantified (n = 3 Western analyses from separate protein extractions; mean ± SEM). Students t-test, *p<0.05.
Figure 6. Arr-HSC cell spreading is impaired in the presence of a function-blocking antibody to α1 integrin. A.
The impedance, reflecting cell adhesion and spreading, was measured for Ctrl-HSC and Arr-HSC cells using electric cell-substrate impedance sensing (ECIS) (mean of duplicate wells of representative ECIS plates). The Arr-HSC cells showed markedly higher impedance at a low frequency than the control cells. B. Treatment of Arr-HSC cells with a specific function-blocking α1 antibody reduced the impedance when compared to the untreated Arr-HSC cells. Treatment of Arr-HSC cells with integrin α2 antibody almost completely abolished the cell spreading. C. Ctrl-HSC cells showed reduced spreading in the presence of integrin α2 antibody while α1 antibody had no effect on impedance.
Similar articles
- Endostatin induces proliferation of oral carcinoma cells but its effect on invasion is modified by the tumor microenvironment.
Alahuhta I, Aikio M, Väyrynen O, Nurmenniemi S, Suojanen J, Teppo S, Pihlajaniemi T, Heljasvaara R, Salo T, Nyberg P. Alahuhta I, et al. Exp Cell Res. 2015 Aug 1;336(1):130-40. doi: 10.1016/j.yexcr.2015.06.012. Epub 2015 Jun 22. Exp Cell Res. 2015. PMID: 26112215 - Characterization of the anti-angiogenic properties of arresten, an alpha1beta1 integrin-dependent collagen-derived tumor suppressor.
Nyberg P, Xie L, Sugimoto H, Colorado P, Sund M, Holthaus K, Sudhakar A, Salo T, Kalluri R. Nyberg P, et al. Exp Cell Res. 2008 Nov 1;314(18):3292-305. doi: 10.1016/j.yexcr.2008.08.011. Epub 2008 Aug 26. Exp Cell Res. 2008. PMID: 18775695 Free PMC article. - Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin.
Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. Sudhakar A, et al. J Clin Invest. 2005 Oct;115(10):2801-10. doi: 10.1172/JCI24813. Epub 2005 Sep 8. J Clin Invest. 2005. PMID: 16151532 Free PMC article. Retracted. - Inhibition of tumor angiogenesis by tumstatin: insights into signaling mechanisms and implications in cancer regression.
Sudhakar A, Boosani CS. Sudhakar A, et al. Pharm Res. 2008 Dec;25(12):2731-9. doi: 10.1007/s11095-008-9634-z. Epub 2008 Jun 13. Pharm Res. 2008. PMID: 18551250 Free PMC article. Retracted. Review. - Discovery of type IV collagen non-collagenous domains as novel integrin ligands and endogenous inhibitors of angiogenesis.
Kalluri R. Kalluri R. Cold Spring Harb Symp Quant Biol. 2002;67:255-66. doi: 10.1101/sqb.2002.67.255. Cold Spring Harb Symp Quant Biol. 2002. PMID: 12858548 Review. No abstract available.
Cited by
- The Versatility of Collagen in Pharmacology: Targeting Collagen, Targeting with Collagen.
Revert-Ros F, Ventura I, Prieto-Ruiz JA, Hernández-Andreu JM, Revert F. Revert-Ros F, et al. Int J Mol Sci. 2024 Jun 13;25(12):6523. doi: 10.3390/ijms25126523. Int J Mol Sci. 2024. PMID: 38928229 Free PMC article. Review. - Understanding the matrix: collagen modifications in tumors and their implications for immunotherapy.
Borst R, Meyaard L, Pascoal Ramos MI. Borst R, et al. J Transl Med. 2024 Apr 24;22(1):382. doi: 10.1186/s12967-024-05199-3. J Transl Med. 2024. PMID: 38659022 Free PMC article. Review. - An arresten-derived anti-angiogenic peptide triggers apoptotic cell death in endothelial cells.
Chamani R, Saberi O, Fathinejad F. Chamani R, et al. Mol Biol Rep. 2024 Apr 15;51(1):513. doi: 10.1007/s11033-024-09448-y. Mol Biol Rep. 2024. PMID: 38622345 - Matrikines in kidney ageing and age-related disease.
Eckersley A, Yamamura T, Lennon R. Eckersley A, et al. Curr Opin Nephrol Hypertens. 2023 Nov 1;32(6):551-558. doi: 10.1097/MNH.0000000000000916. Epub 2023 Aug 16. Curr Opin Nephrol Hypertens. 2023. PMID: 37584348 Free PMC article. Review. - DeepsmirUD: Prediction of Regulatory Effects on microRNA Expression Mediated by Small Molecules Using Deep Learning.
Sun J, Ru J, Ramos-Mucci L, Qi F, Chen Z, Chen S, Cribbs AP, Deng L, Wang X. Sun J, et al. Int J Mol Sci. 2023 Jan 18;24(3):1878. doi: 10.3390/ijms24031878. Int J Mol Sci. 2023. PMID: 36768205 Free PMC article.
References
- Nyberg P, Xie L, Kalluri R (2005) Endogenous inhibitors of angiogenesis. Cancer Res 65: 3967–3979. - PubMed
- Nyberg P, Salo T, Kalluri R (2008) Tumor microenvironment and angiogenesis. Front Biosci 13: 6537–6553. - PubMed
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, et al. (2000) Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 60: 2520–2526. - PubMed
- Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, et al. (2000) Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem 275(2): 1209–1215. - PubMed
- Pasco S, Ramont L, Venteo L, Pluot M, Maquart FX, et al. (2004) In vivo overexpression of tumstatin domains by tumor cells inhibits their invasive properties in a mouse melanoma model. Exp Cell Res 301(2): 251–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was financially supported by Academy of Finland (1126783, 1130140, 115256, 128259, 132051, 138866), Centre of Excellence of Academy of Finland Grant 2012–2017 (251314, Finnish Cancer Organizations, Cancer Foundation of Northern Finland, Sigrid Juselius Foundation, Oulu and Helsinki University Hospital KEVO-support, Oulu University Scholarship Foundation, and Finnish Dental Society Apollonia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous