Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis - PubMed (original) (raw)
. 2012 Dec 14;18(46):6819-28.
doi: 10.3748/wjg.v18.i46.6819.
Yuan Lu, Lu-Yi Wu, Shu-Guang Yu, Bai-Xiao Zhao, Hong-Yi Hu, Huan-Gan Wu, Chun-Hui Bao, Hui-Rong Liu, Jin-Hai Wang, Yi Yao, Xue-Gui Hua, Hui-Ying Guo, Li-Rong Shen
Affiliations
- PMID: 23239920
- PMCID: PMC3520171
- DOI: 10.3748/wjg.v18.i46.6819
Moxibustion inhibits interleukin-12 and tumor necrosis factor alpha and modulates intestinal flora in rat with ulcerative colitis
Xiao-Mei Wang et al. World J Gastroenterol. 2012.
Abstract
Aim: To investigate the effect of moxibustion on intestinal flora and release of interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α) from the colon in rat with ulcerative colitis (UC).
Methods: A rat model of UC was established by local stimulation of the intestine with supernatant from colonic contents harvested from human UC patients. A total of 40 male Sprague-Dawley rats were randomly divided into the following groups: normal (sham), model (UC), herb-partition moxibustion (HPM-treated), and positive control sulfasalazine (SA-treated). Rats treated with HPM received HPM at acupuncture points ST25 and RN6, once a day for 15 min, for a total of 8 d. Rats in the SA group were perfused with SA twice a day for 8 d. The colonic histopathology was observed by hematoxylin-eosin. The levels of intestinal flora, including Bifidobacterium, Lactobacillus, Escherichia coli (E. coli), and Bacteroides fragilis (B. fragilis), were tested by real-time quantitative polymerase chain reaction to detect bacterial 16S rRNA/DNA in order to determine DNA copy numbers of each specific species. Immunohistochemical assays were used to observe the expression of TNF-α and IL-12 in the rat colons.
Results: HPM treatment inhibited immunopathology in colonic tissues of UC rats; the general morphological score and the immunopathological score were significantly decreased in the HPM and SA groups compared with the model group [3.5 (2.0-4.0), 3.0 (1.5-3.5) vs 6.0 (5.5-7.0), P < 0.05 for the general morphological score, and 3.00 (2.00-3.50), 3.00 (2.50-3.50) vs 5.00 (4.50-5.50), P < 0.01 for the immunopathological score]. As measured by DNA copy number, we found that Bifidobacterium and Lactobacillus, which are associated with a healthy colon, were significantly higher in the HPM and SA groups than in the model group (1.395 ± 1.339, 1.461 ± 1.152 vs 0.045 ± 0.036, P < 0.01 for Bifidobacterium, and 0.395 ± 0.325, 0.851 ± 0.651 vs 0.0015 ± 0.0014, P < 0.01 for Lactobacillus). On the other hand, E. coli and B. fragilis, which are associated with an inflamed colon, were significantly lower in the HPM and SA groups than in the model group (0.244 ± 0.107, 0.628 ± 0.257 vs 1.691 ± 0.683, P < 0.01 for E. coli, and 0.351 ± 0.181, 0.416 ± 0.329 vs 1.285 ± 1.039, P < 0.01 for B. fragilis). The expression of TNF-α and IL-12 was decreased after HPM and SA treatment as compared to UC model alone (4970.81 ± 959.78, 6635.45 ± 1135.16 vs 12333.81 ± 680.79, P < 0.01 for TNF-α, and 5528.75 ± 1245.72, 7477.38 ± 1259.16 vs 12550.29 ± 1973.30, P < 0.01 for IL-12).
Conclusion: HPM treatment can regulate intestinal flora and inhibit the expression of TNF-α and IL-12 in the colon tissues of UC rats, indicating that HPM can improve colonic immune response.
Keywords: Herb-partition moxibustion; Immune regulation; Intestinal flora; Ulcerative colitis.
Figures
Figure 1
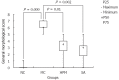
Herb-partition moxibustion inhibits tissue damage in colonic tissues of rats with ulcerative colitis. The general morphological score of the colonic tissue in the model control (MC) group was significantly higher than the normal control (NC) group (P = 0.000). After treatment, the scores were lower in both the herb-partition moxibustion (HPM) group (P = 0.01) and the control sulfasalazine (SA) group (P = 0.002).
Figure 2
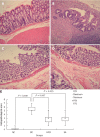
Herb-partition moxibustion inhibits immunopathology and decreases the histopathological scores in colonic tissues of rats with ulcerative colitis. A: Normal; B: Ulcerative colitis (UC); C: Herb-partition moxibustion (HPM); D: Sulfasalazine (SA); E: The histopathological scores for the colonic tissue in the UC model control (MC) group were significantly higher than the normal control (NC) group (P = 0.000). After treatment, the scores were lower in both the HPM group (P = 0.007) and SA group (P = 0.015). The colonic mucosa was damaged in the UC model group with cell infiltration, congestion, edema, and ulceration. After HPM treatment, there are only slight submucosal edema and inflammatory cell infiltration, and the colonic mucosa epithelium and the colonic gland were more regularly arranged than in the model group, new epithelial cells were observed to be covering the ulcers. Positive control SA treatment showed similar recovery of the UC model as the HPM group.
Figure 3
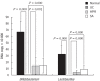
Herb-partition moxibustion treatment increases the colonic Bifidobacterium and Lactobacillus of rats with ulcerative colitis. The DNA copies of the symbiotic groups Bifidobacterium and Lactobacillus were both significantly decreased in the ulcerative colitis (UC) group compared to the normal control (NC) group (P = 0.000). After herb-partition moxibustion (HPM) treatment, the DNA copies of Bifidobacterium and Lactobacillus were both significantly increased in the HPM group (P = 0.001 and P = 0.000) and sulfasalazine (SA) group (P = 0.000 and P = 0.000) compared with the UC model group.
Figure 4
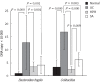
Herb-partition moxibustion treatment decreases the colonic pathogenic bacteria Bacteroides fragilis and Escherichia coli of rats with ulcerative colitis. The DNA copies of the pathogenic bacteria Bacteroides fragilis (B. fragilis), and Escherichia coli (E. coli) were both significantly increased in the ulcerative colitis (UC) rats compared to the normal control (NC) rats (P = 0.000). After herb-partition moxibustion (HPM) treatment, the DNA copies of B. fragilis and E. coli were both significantly decreased in the HPM group (P = 0.001 and P = 0.000) and sulfasalazine (SA) group (P = 0.001 and P = 0.003) compared with the UC model group.
Similar articles
- Herb-partitioned moxibustion alleviates colon injuries in ulcerative colitis rats.
Zhang D, Ren YB, Wei K, Hong J, Yang YT, Wu LJ, Zhang J, Shi Z, Wu HG, Ma XP. Zhang D, et al. World J Gastroenterol. 2018 Aug 14;24(30):3384-3397. doi: 10.3748/wjg.v24.i30.3384. World J Gastroenterol. 2018. PMID: 30122878 Free PMC article. - Moxibustion treatment modulates the gut microbiota and immune function in a dextran sulphate sodium-induced colitis rat model.
Qi Q, Liu YN, Jin XM, Zhang LS, Wang C, Bao CH, Liu HR, Wu HG, Wang XM. Qi Q, et al. World J Gastroenterol. 2018 Jul 28;24(28):3130-3144. doi: 10.3748/wjg.v24.i28.3130. World J Gastroenterol. 2018. PMID: 30065559 Free PMC article. - Mechanism of Acupuncture and Moxibustion on Promoting Mucosal Healing in Ulcerative Colitis.
Li H, Ye XF, Su YS, He W, Zhang JB, Zhang Q, Zhan LB, Jing XH. Li H, et al. Chin J Integr Med. 2023 Sep;29(9):847-856. doi: 10.1007/s11655-022-3531-x. Epub 2022 Apr 12. Chin J Integr Med. 2023. PMID: 35412218 Review.
Cited by
- Intestinal Microbiota-Associated Metabolites: Crucial Factors in the Effectiveness of Herbal Medicines and Diet Therapies.
Wang Y, Qin S, Jia J, Huang L, Li F, Jin F, Ren Z, Wang Y. Wang Y, et al. Front Physiol. 2019 Oct 29;10:1343. doi: 10.3389/fphys.2019.01343. eCollection 2019. Front Physiol. 2019. PMID: 31736775 Free PMC article. Review. - Electroceuticals and Magnetoceuticals in Gastroenterology.
Song G, Sclocco R, Sharma A, Guerrero-López I, Kuo B. Song G, et al. Biomolecules. 2024 Jun 26;14(7):760. doi: 10.3390/biom14070760. Biomolecules. 2024. PMID: 39062474 Free PMC article. Review. - Regulating the Balance of Th17/Treg via Electroacupuncture and Moxibustion: An Ulcerative Colitis Mice Model Based Study.
Sun J, Zhang H, Wang C, Yang M, Chang S, Geng Y, Yang H, Zhuang Z, Wang X, Xie L, Huang B, Zhao N, Zhou W, Cheng X, Cai B, Wu Q, Yu SG. Sun J, et al. Evid Based Complement Alternat Med. 2017;2017:7296353. doi: 10.1155/2017/7296353. Epub 2017 Dec 17. Evid Based Complement Alternat Med. 2017. PMID: 29391874 Free PMC article. - Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome.
Bao CH, Wang CY, Li GN, Yan YL, Wang D, Jin XM, Wu LY, Liu HR, Wang XM, Shi Z, Wu HG. Bao CH, et al. World J Gastroenterol. 2019 Aug 28;25(32):4696-4714. doi: 10.3748/wjg.v25.i32.4696. World J Gastroenterol. 2019. PMID: 31528095 Free PMC article. - Effects of Different Local Moxibustion-Like Stimuli at Zusanli (ST36) and Zhongwan (CV12) on Gastric Motility and Its Underlying Receptor Mechanism.
Su YS, Xin JJ, Yang ZK, He W, Shi H, Wang XY, Hu L, Jing XH, Zhu B. Su YS, et al. Evid Based Complement Alternat Med. 2015;2015:486963. doi: 10.1155/2015/486963. Epub 2015 Jul 12. Evid Based Complement Alternat Med. 2015. PMID: 26246837 Free PMC article.
References
- Watanabe S, Yamakawa M, Hiroaki T, Kawata S, Kimura O. Correlation of dendritic cell infiltration with active crypt inflammation in ulcerative colitis. Clin Immunol. 2007;122:288–297. - PubMed
- Zhang SZ, Zhang DC. Immunological mechanism of ulcerative colitis. Zhongguo Jianyan Yixue Zazhi. 2006;27:419–421.
- Macfarlane S, Furrie E, Kennedy A, Cummings JH, Macfarlane GT. Mucosal bacteria in ulcerative colitis. Br J Nutr. 2005;93 Suppl 1:S67–S72. - PubMed
- Andoh A, Imaeda H, Aomatsu T, Inatomi O, Bamba S, Sasaki M, Saito Y, Tsujikawa T, Fujiyama Y. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials