Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain - PubMed (original) (raw)
doi: 10.1161/ATVBAHA.112.300485. Epub 2012 Dec 13.
Yi Guo, Espen J Walker, Fanxia Shen, Kristine Jun, S Paul Oh, Vincent Degos, Michael T Lawton, Tarik Tihan, Dimitrios Davalos, Katerina Akassoglou, Jeffrey Nelson, John Pile-Spellman, Hua Su, William L Young
Affiliations
- PMID: 23241407
- PMCID: PMC3569037
- DOI: 10.1161/ATVBAHA.112.300485
Reduced mural cell coverage and impaired vessel integrity after angiogenic stimulation in the Alk1-deficient brain
Wanqiu Chen et al. Arterioscler Thromb Vasc Biol. 2013 Feb.
Abstract
Objective: Vessels in brain arteriovenous malformations are prone to rupture. The underlying pathogenesis is not clear. Hereditary hemorrhagic telangiectasia type 2 patients with activin receptor-like kinase 1 (Alk1) mutation have a higher incidence of brain arteriovenous malformation than the general population. We tested the hypothesis that vascular endothelial growth factor impairs vascular integrity in the Alk1-deficient brain through reduction of mural cell coverage.
Methods and results: Adult Alk1(1f/2f) mice (loxP sites flanking exons 4-6) and wild-type mice were injected with 2×10(7) PFU adenovious-cre recombinase and 2×10(9) genome copies of adeno-associated virus-vascular endothelial growth factor to induce focal homozygous Alk1 deletion (in Alk1(1f/2f) mice) and angiogenesis. Brain vessels were analyzed 8 weeks later. Compared with wild-type mice, the Alk1-deficient brain had more fibrin (99±30×10(3) pixels/mm(2) versus 40±13×10(3); P=0.001), iron deposition (508±506 pixels/mm(2) versus 6±49; P=0.04), and Iba1(+) microglia/macrophage infiltration (888±420 Iba1(+) cells/mm(2) versus 240±104 Iba1(+); P=0.001) after vascular endothelial growth factor stimulation. In the angiogenic foci, the Alk1-deficient brain had more α-smooth muscle actin negative vessels (52±9% versus 12±7%, P<0.001), fewer vascular-associated pericytes (503±179/mm(2) versus 931±115, P<0.001), and reduced platelet-derived growth factor receptor-β expression.
Conclusions: Reduction of mural cell coverage in response to vascular endothelial growth factor stimulation is a potential mechanism for the impairment of vessel wall integrity in hereditary hemorrhagic telangiectasia type 2-associated brain arteriovenous malformation.
Figures
Figure 1
The dysplastic vessels in the angiogenic foci of _Alk1_-deficient brain have increased permeability than vessels in the angiogenic foci of the normal brain. (A) Representative images show vessels (lectin, green) and fibrin (red) in the viral-injected region. (B) High magnification images show the location of fibrin. The fibrin lines outside the dysplastic vessel wall in the _Alk1_-deficient brain. (C) Bar graph shows the quantification of fibrin. In the viral-injected region, there were significantly more fibrin deposition in the VEGF-treated _Alk1_-deficient brain than VEGF-treated WT (*p= 0.001) and _Alk1_2f/2f (*p= 0.003) groups, and _Alk1_-deficient brain without VEGF treatment (*p<0.001). Scale bars: 50 μm.
Figure 2
RBCs and iron deposition were detected around the dysplastic vessels. (A) Representative images of Prussian blue staining show the iron deposition around dysplastic vessels in the angiogenic foci of _Alk1_-deficient brain. (B) Bar graph shows the quantification of Prussian blue-positive area. The VEGF-stimulated _Alk1_-deficient group had significantly increased Prussian blue-positive area than VEGF-treated WT (*p=0.04) and _Alk1_2f/2f (*p=0.03) group, and untreated _Alk1_-deficient brain (*p=0.02). (C) RBCs were found in extravascular space (black arrow). Scale bar: 50 μm. The insert shows a macrophage with three red blood cells in its cytoplasm. Scale bar in the insert: 10 μm. The brain was perfused with PBS before collection. (D) Representative images of hematoxylin and eosin (H&E) and Prussian blue stained _Alk1_-deficient mouse and human bAVM sections. Hemosiderin and Prussian blue-positive staining were detected around dysplastic vessels in the angiogenic foci of _Alk1_-deficient brain and in the human bAVM. Scale bars: 50 μm.
Figure 3
Prussian blue staining positively correlated with macrophage/microglia infiltration. (A) Representative images of Iba1 antibody staining (red). Scale bar: 50 μm. (B) Bar graph shows quantification of Iba1+ cells. The VEGF-stimulated _Alk1_-deficient group had more Iba1+ cells than VEGF-treated WT (*p=0.001) and _Alk1_2f/2f (*p=0.015) groups, and untreated _Alk1_-deficient-group (*p=0.002). (C) Graph shows Prussian blue-positive area positively correlated with the number of Iba1+ cells (R2=0.2, *p<0.05).
Figure 4
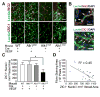
The dysplastic vessels in the angiogenic foci of _Alk1_-deficient brain had less coverage of α-SMA+ cells. (A) Representative images of lectin (green) and anti-αSMA (red) stained sections. In the angiogenic foci of _Alk1_-deficient brain, some vessels larger than 15 μm had no α-SMA+ cells (white arrows). Scale bar: 50 μm. (B) Bar graph shows the percentage of α-SMA- vessels (>15 μm). Significantly more α-SMA- vessels presented in the VEGF-stimulated _Alk1_-deficient brain than that in WT (*p<0.001), _Alk1_2f/2f (*p<0.001) and _Alk1_-deficient-only (*p<0.001) groups. (C) Prussian Blue stains were detected around α-SMA- vessels. The left panel shows Prussian blue-positive staining around the α-SMA- vessels, such as Vessels a and b (black arrows). Scale bars: 50 μm. The right panel shows high magnification images of Vessel a from the left panel. H&E (top) and Prussian blue (bottom) stained sections. Brown colored cells around vessels are hemosiderin positive macrophages. Scale bars: 30 μm. (D) Pie graphs show significantly more PB+ vessels are α-SMA- vessels (*p<0.001). PB: Prussian blue.
Figure 5
The dysplastic vessels in the angiogenic foci of _Alk1_-deficient brain have fewer pericytes on their walls. (A) Representative images show vessels (lectin, green) and pericytes (upper panel: NG2, red; lower panel: vascular associated ZIC1+ cells, red) in the vector injection sites. * in upper panel and arrows in lower panel indicate dysplastic vessels. Scale bars: 50 μm. (B) High magnification images of pericytes. The upper panel shows vascular (green) associated NG2+ pericyte (arrows, red). The lower panel shows the ZIC1+ pericytes (arrows, red) co-localized with nuclei (DAPI, blue). Scale bars: 20 μm. (C) Bar graph shows the quantification of pericytes located on the vessel wall. (D) Graph shows the number of vessel-associated pericyte inversely correlating with the degree of fibrin deposition (R2 =0.45).
References
- Attia W, Tada T, Hongo K, Nagashima H, Takemae T, Tanaka Y, Kobayashi S. Microvascular pathological features of immediate perinidal parenchyma in cerebral arteriovenous malformations: giant bed capillaries. J Neurosurg. 2003;98:823–827. - PubMed
- Sato S, Kodama N, Sasaki T, Matsumoto M, Ishikawa T. Perinidal dilated capillary networks in cerebral arteriovenous malformations. Neurosurgery. 2004;54:163–168. discussion 168-170. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 HL064024/HL/NHLBI NIH HHS/United States
- P01 NS044155/NS/NINDS NIH HHS/United States
- R01 NS027713/NS/NINDS NIH HHS/United States
- GM008440/GM/NIGMS NIH HHS/United States
- T32 GM008440/GM/NIGMS NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- R21 NS070153/NS/NINDS NIH HHS/United States
- R01HL64024/HL/NHLBI NIH HHS/United States
- R01 NS066361/NS/NINDS NIH HHS/United States
- R01 NS052189/NS/NINDS NIH HHS/United States
- R01 NS051470/NS/NINDS NIH HHS/United States
- R01 HL122774/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases