Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii - PubMed (original) (raw)
Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii
A Alicia Koblansky et al. Immunity. 2013.
Abstract
Toll-like receptor 11 (TLR11) recognizes T. gondii profilin (TgPRF) and is required for interleukin-12 production and induction of immune responses that limit cyst burden in Toxoplasma gondii-infected mice. However, TLR11 only modestly affects survival of T. gondii-challenged mice. We report that TLR12, a previously uncharacterized TLR, also recognized TgPRF. TLR12 was sufficient for recognition of TgPRF by plasmacytoid dendritic cells (pDCs), whereas TLR11 and TLR12 were both required in macrophages and conventional DCs. In contrast to TLR11, TLR12-deficient mice succumb rapidly to T. gondii infection. TLR12-dependent induction of IL-12 and IFN-α in pDCs led to production of IFN-γ by NK cells. Consistent with this observation, the partial resistance of Tlr11(-/-) mice is lost upon pDC or NK cell depletion. Thus, TLR12 is critical for the innate immune response to T. gondii, and this TLR may promote host resistance by triggering pDC and NK cell function.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
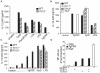
Figure 1. TLR12 Can Form Homodimers and Heterodimers with TLR11 but Has a More Restricted Expression Pattern
(A) Alignment of amino acid sequences of TLR12 and TLR11. The transmembrane region is boxed and darker gray shading indicates amino acid residues that are identical. (B) Phylogenetic analysis of known TLRs with TLR12 was performed with CLUSTALW software with the fullamino acid sequences of murine TLRs 1–9 and TLRs11–12. (C) RT-PCR was used to determine TLR11 and TLR12 mRNA levels in multiple tissues and purified hematopoietic cell populations. A β-actin probe was used as a control for RNA loading. NTC, no template control. Data are representative of three independent experiments. (D) TLR12 can form homodimers with itself and a heterodimer with TLR11. Flag-TLR12 and HA-TLR12 were transfected into HEK293 cells. Cells were lysed and coimunoprecipitation with either anti-flag or anti-HA, followed by SDS-PAGE and protein blot analysis with antibodies specific for Flag and HA. Data are representative of three independent experiments. (E) TLR12 and TLR11 interact with Unc-93B1. HEK293 cells were transiently transfected with TLR12-Flag or TLR11-Flag and Unc-93B1-GFP as indicated, lysed, and subjected to anti-flag immunoprecipitation, SDS-PAGE, and protein blotting with antibodies specific for Flag and GFP. Data are representative of two independent experiments. See also Figures S1 and S2.
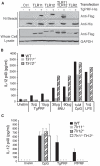
Figure 2. TLR12 Recognizes TgPRF and Is Required for Production of IL-12 in Macrophages and CD11c+ Cells
(A) Thioglycollate-elicited peritoneal macrophages were isolated from wild-type control (WT), _Tlr11_−/−, and _Tlr12_−/− mice and were stimulated with 10 ng/ml LPS, 1 μM CpG, 10 μg/ml LTA, 10 μg/ml Pam3CSK4, or 1.5 μg/ml TgPRF for 24 hr. The concentrations of IL-12p40 in the supernatants were measured by ELISA. (B) CD11c+ splenic cells from WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr4_−/− mice were stimulated with 1 μM CpG or 2 μg/ml TgPRF or 10 ng/ml LPS for 24 hr and concentrations of IL-12p40 was measured by ELISA (asterisk indicates values below the limit of detection). (C) CD11c+ splenic cells from WT, _Tlr11_−/−, and _Tlr12_−/− mice were stimulated with either CpG or increasing amounts of TgPRF (0.25–25 μg/ml) for 24 hr, and the concentrations of IL-12p40 was measured by ELISA. (D) Recognition of TgPRF by either TLR11 or TLR12 can induce NF-κB activity. HEK293 cells stably expressing an NF-κB luciferase reporter construct were transfected with vectors encoding Flag-TLR11 or Flag-TLR12 and later stimulated with 6 μg/ml of TgPRF or 10 ng/ml of TNF as indicated, for 7 hr. n = 3; error bars are plus and minus one standard deviation [SD]; data are representative of three independent experiments. See also Figure S3.
Figure 3. TLR12 Specifically Recognizes Profilin from Apicomplexan Parasites
(A) Both TLR11 and TLR12 can pull down TgPRF, either as homodimers or heterodimers. HEK293 cells transiently expressing Flag-TLR12 and Flag-TLR11 were lysed and incubated with His-TgPRF. His-TgPRF was subjected to Ni-affinity resin pull down and analyzed for TLR12 and TLR11 binding by protein blotting with antibodies specific for Flag and then His. (B) _Tlr12_−/− cells can respond to stimulation by uropathogenic E. coli 8NU. CD11c+ splenocytes were isolated from WT, _Tlr11_−/−, and _Tlr12_−/− and stimulated overnight with TgPRF, 8NU, and LPS. Amounts of IL-12p40 in the supernatants were measured by ELISA (n = 3; error bars are plus and minus 1 SD). (C) TLR12 can recognize profilin from Plasmodium falciparum. Thioglycollate-elicited peritoneal macrophages from wild-type (WT), _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice were stimulated for 24 hr with 1.0 μM of CpG, 3.0 μg/ml of TgPRF, and 3.0 μg/ml of P. falciparum profilin (PfPRF). After 24 hr supernatant was removed, and IL-12(p40) was measured by ELISA (n = 3; error bars are plus and minus 1 SD; asterisk indicates values below the limit of detection). Data in all panels are representative of at least three independent experiments.
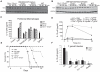
Figure 4. TLR12 Is Required for Protection against T. gondii Infection
(A and B) TLR11 and TLR12 are required for optimal NF-κB activation in response to TgPRF stimulation of (A) bone marrow-derived dendritic cell culture (GM-CSF) and (B) bone-marrow-derived macrophages isolated from WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice. GM-CSF cultures were stimulated with 3 μg/ml of TgPRF for indicated times and bone-marrow-derived macrophages were stimulated with 6 μg/ml of TgPRF. Cells were harvested and analyzed by protein blotting for IκBα degradation. Data are representative of two independent experiments. (C) _Tlr11_−/−_Tlr12_−/− thioglycollate-elicited peritoneal macrophages fail to produce IL-12 in response to stimulation from PFTG. Macrophages from WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice were isolated and stimulated with 10 ng/ml LPS, 500 μM CpG, 10 μg/ml LTA, 10 μg/ml Pam3CSK4, and 1.5 μg/ml TgPRF for 24 hr. The levels of IL-12p40 in the supernatants were measured by ELISA (n = 3; error bars are plus and minus 1 SD; asterisk indicates values below the limit of detection; representative of three independent experiments). (D) Low levels of IL-12p40 are detected in the serum of _Tlr11_−/− and _Tlr12_−/− after injection with TgPRF. WT, _Tlr11_−/−, _Tlr12_−/− mice (n = 3 per group) received intraperitoneal injection with 10 μg/ml TgPRF and were bled at the time points indicated. Serum levels of IL-12(p40) were measured by ELISA (n = 3; error bars are plus and minus 1 SD; representative of three independent experiments). (E) _Myd88_−/−, _Tlr11_−/−_Tlr12_−/−, and _Tlr12_−/− mice succumb to T. gondii infection. WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice were infected with 20 cysts of T. gondii (ME49 strain) and monitored for survival (representative of three independent experiments). (F) _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice have significantly lower serum IL-12 compared to WT mice during T. gondii infection. Measurement of serum IL-12 by ELISA from infected animals at post infection days 5 and 7 (error bars are plus and minus 1 SD; representative of three independent experiments).
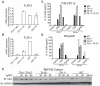
Figure 5. TLR12, but Not TLR11, Mediates Recognition of TgPRF and Production of IL-12 by pDCs
(A and B) Quantitative PCR analysis of TLR12 (A) and TLR11 (B) mRNA in total CD11c+ conventional DCs, plasmacytoid dendritic cells (pDC; Singlec-HhiCD11cint), CD8α+ dendritic cells (CD8α+; CD11chi, MHC class II+, CD8α+ population), and CD4+ T cells. PCR results were normalized with primers specific for β-actin. (C) FACS-sorted CD11c+ Flt3L BMDCs were stimulated with either 1 μM CpG or 3 μg/ml TgPRF for 24 hr. Supernatants were collected and analyzed by IL-12 ELISA. (D) FACS-sorted pDCs from Flt3L BMDC cultures were stimulated with CpG or TgPRF. Supernatant IL-12p40 was analyzed by ELISA. (E) Protein blotting analysis of Flt3L BMDC culture reveals that only TLR12 is required for NF-κB activation. Flt3L BMDCs isolated from WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice were stimulated with 3 μg/ml of TgPRF for indicated times. Cells were harvested, lysed, and analyzed by protein blotting for IκBα degradation. Error bars are plus and minus 1 SD; data representative of three independent experiments.
Figure 6. TLR12-Dependent Production of IL-12 and IFN-α Is Required for Activation of NK Cells and Production of IFN-γ
(A) TgPRF induction of IL-12 in pDCs is TLR11 independent but TLR12 and MyD88 dependent. Flt3L BMDC cultures from WT, _Tlr11_−/−, _Tlr12_−/−, _Tlr11_−/−_Tlr12_−/−, and _Myd88_−/− mice were stimulated with 1 μM CpG and 3 μg/ml TgPRF for 24 hr, and supernatants were analyzed by ELISA (error bars are plus and minus 1 SD; representative of three independent experiments). (B) TgPRF stimulation can induce IFN-α in primary CD11c+ splenocytes. Cells were stimulated with either CpG or TgPRF for 48 hr. Supernatants were analyzed after 24 hr by ELISA (error bars are plus and minus 1 SD; representative of three independent experiments). (C) WT, _Tlr11_−/−, _Tlr12_−/−, and _Tlr11_−/−_Tlr12_−/− mice were injected intraperitoneally with 4 μg/ml TgPRF and single-cell suspension of splenocytes were prepared. Splenocytes were stained for anti-NK1.1 and anti-IFN-γ and analyzed by flow cytometry. (D) Histograms showing amounts of intercellular IFN-γ in NK1.1+ cells after TgPRF injection. The black lines represent PBS-injected and the red line represents TgPRF-injected animals. Data are representative of at least two independent experiments. (E) _Tlr11_−/− mice were injected i.p. with either anti-CD317 (mAb 927) or control ascites (0.5 μg Ig/mouse) or i.v. with 50 μl of anti-AsialoGM1 on day −1 and +3 relative to infection, and their survival was monitored daily. See also Figure S4.
Similar articles
- Cooperation of TLR12 and TLR11 in the IRF8-dependent IL-12 response to Toxoplasma gondii profilin.
Raetz M, Kibardin A, Sturge CR, Pifer R, Li H, Burstein E, Ozato K, Larin S, Yarovinsky F. Raetz M, et al. J Immunol. 2013 Nov 1;191(9):4818-27. doi: 10.4049/jimmunol.1301301. Epub 2013 Sep 27. J Immunol. 2013. PMID: 24078692 Free PMC article. - TLR11-independent inflammasome activation is critical for CD4+ T cell-derived IFN-γ production and host resistance to Toxoplasma gondii.
López-Yglesias AH, Camanzo E, Martin AT, Araujo AM, Yarovinsky F. López-Yglesias AH, et al. PLoS Pathog. 2019 Jun 13;15(6):e1007872. doi: 10.1371/journal.ppat.1007872. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31194844 Free PMC article. - Naturally occurring Toll-like receptor 11 (TLR11) and Toll-like receptor 12 (TLR12) polymorphisms are not associated with Toxoplasma gondii infection in wild wood mice.
Morger J, Bajnok J, Boyce K, Craig PS, Rogan MT, Lun ZR, Hide G, Tschirren B. Morger J, et al. Infect Genet Evol. 2014 Aug;26:180-4. doi: 10.1016/j.meegid.2014.05.032. Epub 2014 Jun 6. Infect Genet Evol. 2014. PMID: 24910107 - Toll-like receptor recognition of Toxoplasma gondii.
Yarovinsky F, Sher A. Yarovinsky F, et al. Int J Parasitol. 2006 Mar;36(3):255-9. doi: 10.1016/j.ijpara.2005.12.003. Epub 2006 Jan 13. Int J Parasitol. 2006. PMID: 16476433 Review. - Innate responses to Toxoplasma gondii in mice and humans.
Pifer R, Yarovinsky F. Pifer R, et al. Trends Parasitol. 2011 Sep;27(9):388-93. doi: 10.1016/j.pt.2011.03.009. Trends Parasitol. 2011. PMID: 21550851 Free PMC article. Review.
Cited by
- Toxoplasma gondii ROP5 Enhances Type I IFN Responses by Promoting Ubiquitination of STING.
Jin QW, Yu T, Pan M, Fan YM, Ge CC, He XB, Gong JZ, Tao JP, Fu BQ, Jing ZZ, Huang SY. Jin QW, et al. Int J Mol Sci. 2024 Oct 19;25(20):11262. doi: 10.3390/ijms252011262. Int J Mol Sci. 2024. PMID: 39457045 Free PMC article. - Caspase-1 in Cx3cr1-expressing cells drives an IL-18-dependent T cell response that promotes parasite control during acute Toxoplasma gondii infection.
Babcock IW, Sibley LA, Labuzan SA, Cowan MN, Sethi I, Alemu S, Kelly AG, Kovacs MA, Lukens JR, Harris TH. Babcock IW, et al. PLoS Pathog. 2024 Oct 24;20(10):e1012006. doi: 10.1371/journal.ppat.1012006. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39446964 Free PMC article. - T lymphocyte-dependent IL-10 down-regulates a cytokine storm driven by Toxoplasma gondii GRA24.
Doherty CM, Patterson PR, Emeanuwa JA, Belmares Ortega J, Fox BA, Bzik DJ, Denkers EY. Doherty CM, et al. mBio. 2024 Nov 13;15(11):e0145524. doi: 10.1128/mbio.01455-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440975 Free PMC article. - Receptors on Microglia.
Augusto-Oliveira M, Tremblay MÈ, Verkhratsky A. Augusto-Oliveira M, et al. Adv Neurobiol. 2024;37:83-121. doi: 10.1007/978-3-031-55529-9_6. Adv Neurobiol. 2024. PMID: 39207688 Review. - From periphery to center stage: 50 years of advancements in innate immunity.
Carpenter S, O'Neill LAJ. Carpenter S, et al. Cell. 2024 Apr 25;187(9):2030-2051. doi: 10.1016/j.cell.2024.03.036. Cell. 2024. PMID: 38670064 Review.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
- Biron CA. Interferons alpha and beta as immune regulators—a new look. Immunity. 2001;14:661–664. - PubMed
- Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007;179:1129–1137. - PubMed
Note Added in Proof
Publication types
MeSH terms
Substances
Grants and funding
- R37-33443/PHS HHS/United States
- P30 AR044535/AR/NIAMS NIH HHS/United States
- P30 AR058886-01/AR/NIAMS NIH HHS/United States
- P30 AR058886/AR/NIAMS NIH HHS/United States
- R01 AI059440/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 AI033443/AI/NIAID NIH HHS/United States
- R01-59440/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases