The distribution of phosphorylated tau in spinal cords of Alzheimer's disease and non-demented individuals - PubMed (original) (raw)
Comparative Study
The distribution of phosphorylated tau in spinal cords of Alzheimer's disease and non-demented individuals
Brittany N Dugger et al. J Alzheimers Dis. 2013.
Abstract
Abnormal phosphorylation of the microtubule-associated protein tau develops in selected brain regions in normal aging and becomes widespread throughout the brain in Alzheimer's disease (AD). Braak and others have described the distribution of neurofibrillary tangles and deposition of abnormally phosphorylated tau (p-tau) and correlated this with the progressive cognitive dysfunction in AD. However, to date there have been no comprehensive studies examining abnormally phosphorylated tau deposition in the spinal cord as part of normal aging or AD. We investigated, using immunohistochemical methods, the presence of p-tau in the spinal cord of 46 cases with a clinicopathological diagnosis of AD as well as 37 non-demented aged (ND) individuals lacking any defined central nervous system-related clinicopathological diagnosis. We found the cervical cord segments to be the most frequently affected subdivision (96% AD versus 43% ND), followed by thoracic (69% AD versus 37% ND), lumbar (65% AD versus 27% ND), and sacral (53% AD versus 13% ND). The spinal cord was often affected at early-stage brain disease, with p-tau spinal cord immunoreactivity in 40% of subjects at Braak neurofibrillary stage I; however, there were no cases having spinal cord p-tau that did not have p-tau within the brain. As p-tau immunoreactivity is present within the spinal cords of ND as well as AD subjects, it is likely that the phosphorylation of spinal cord tau occurs in the preclinical stage of AD, prior to dementia. The presence of significant spinal cord p-tau-immunoreactive pathology has important implications for both the pathogenesis and clinical manifestations of AD.
Figures
Fig 1
P-tau immunoreactivity in the spinal cord. Ventral part of the cervical horn of a non-demented control (ND) case (a); near the border of ventral horn and intermediolateral region of the lumbar subdivision of an Alzheimer’s disease (AD) case (b); ventral horn of the lumbar subdivision of an AD case (c); intermediolateral region of the lumbar subdivision of an AD case (d); ventral region of the cervical subdivision of an AD case (e); central canal region of the thoracic subdivision of an AD case (f); subpial region of the lumbar subdivision of an AD case (g); and anterior median fissure region of the thoracic subdivision of an AD case (h). Arrows indicate neuropil threads; # indicates neurofibrillary tangles; * indicates pre-tangles; thorned shaped astrocytes are present in (g–h). Insets in a, b, d, g represent higher magnification of small boxed areas. All photos taken at 40x magnification.
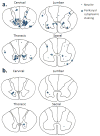
Fig 2
Location and densities of all neurites and perikaryal cytoplasmic staining seen across all analyzed Alzheimer’s disease cases at each level of the spinal cord (a). A representation of the typical distribution and density of neurites and perikaryal cytoplasmic staining on 5μm sections of an individual Alzheimer’s disease case at each level of the spinal cord (b). Each neurite is represented by dot while perikaryal cytoplasmic staining by a star.
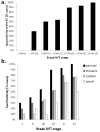
Fig 3
Prevalence (percentage) of p-tau immunoreactivity in subjects by Braak neurofibrillary stage (a) and spinal cord subdivision (b). Both Alzheimer’s disease and non-demented control subjects are included.
Similar articles
- The Presence of Select Tau Species in Human Peripheral Tissues and Their Relation to Alzheimer's Disease.
Dugger BN, Whiteside CM, Maarouf CL, Walker DG, Beach TG, Sue LI, Garcia A, Dunckley T, Meechoovet B, Reiman EM, Roher AE. Dugger BN, et al. J Alzheimers Dis. 2016;51(2):345-56. doi: 10.3233/JAD-150859. J Alzheimers Dis. 2016. PMID: 26890756 Free PMC article. - [Expression of tau-related protein in spinal cord of patients with Alzheimer's disease].
GUO YJ, WANG LN, ZHU MW, ZHANG HH, HU YZ, HAN ZT, LI JM, WANG DX. GUO YJ, et al. Zhonghua Bing Li Xue Za Zhi. 2011 Mar;40(3):161-4. Zhonghua Bing Li Xue Za Zhi. 2011. PMID: 21575385 Chinese. - Soluble pre-fibrillar tau and β-amyloid species emerge in early human Alzheimer's disease and track disease progression and cognitive decline.
Koss DJ, Jones G, Cranston A, Gardner H, Kanaan NM, Platt B. Koss DJ, et al. Acta Neuropathol. 2016 Dec;132(6):875-895. doi: 10.1007/s00401-016-1632-3. Epub 2016 Oct 21. Acta Neuropathol. 2016. PMID: 27770234 Free PMC article. - Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles.
Iqbal K, Alonso AC, Gong CX, Khatoon S, Pei JJ, Wang JZ, Grundke-Iqbal I. Iqbal K, et al. J Neural Transm Suppl. 1998;53:169-80. doi: 10.1007/978-3-7091-6467-9_15. J Neural Transm Suppl. 1998. PMID: 9700655 Review. - The relationship between subcortical tau pathology and Alzheimer's disease.
Attems J, Thal DR, Jellinger KA. Attems J, et al. Biochem Soc Trans. 2012 Aug;40(4):711-5. doi: 10.1042/BST20120034. Biochem Soc Trans. 2012. PMID: 22817721 Review.
Cited by
- The Presence of Select Tau Species in Human Peripheral Tissues and Their Relation to Alzheimer's Disease.
Dugger BN, Whiteside CM, Maarouf CL, Walker DG, Beach TG, Sue LI, Garcia A, Dunckley T, Meechoovet B, Reiman EM, Roher AE. Dugger BN, et al. J Alzheimers Dis. 2016;51(2):345-56. doi: 10.3233/JAD-150859. J Alzheimers Dis. 2016. PMID: 26890756 Free PMC article. - Detection of hyperphosphorylated tau protein and α-synuclein in spinal cord of patients with Alzheimer's disease.
Guo Y, Wang L, Zhu M, Zhang H, Hu Y, Han Z, Liu J, Zhao W, Wang D. Guo Y, et al. Neuropsychiatr Dis Treat. 2016 Feb 26;12:445-52. doi: 10.2147/NDT.S90735. eCollection 2016. Neuropsychiatr Dis Treat. 2016. PMID: 27013875 Free PMC article. - Reversible Tau Phosphorylation Induced by Synthetic Torpor in the Spinal Cord of the Rat.
Hitrec T, Squarcio F, Cerri M, Martelli D, Occhinegro A, Piscitiello E, Tupone D, Amici R, Luppi M. Hitrec T, et al. Front Neuroanat. 2021 Feb 2;15:592288. doi: 10.3389/fnana.2021.592288. eCollection 2021. Front Neuroanat. 2021. PMID: 33603651 Free PMC article. - Primary Age-Related Tauopathy in Human Subcortical Nuclei.
Zhu K, Wang X, Sun B, Wu J, Lu H, Zhang X, Liang H, Zhang D, Liu C. Zhu K, et al. Front Neurosci. 2019 May 29;13:529. doi: 10.3389/fnins.2019.00529. eCollection 2019. Front Neurosci. 2019. PMID: 31191227 Free PMC article. - An Overview of Experimental and Clinical Spinal Cord Findings in Alzheimer's Disease.
Xie Q, Zhao WJ, Ou GY, Xue WK. Xie Q, et al. Brain Sci. 2019 Jul 17;9(7):168. doi: 10.3390/brainsci9070168. Brain Sci. 2019. PMID: 31319495 Free PMC article.
References
- Roberts E. Alzheimer’s disease may begin in the nose and may be caused by aluminosilicates. Neurobiol Aging. 1986;7:561–567. - PubMed
- Lee JH, Goedert M, Hill WD, Lee VM, Trojanowski JQ. Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp Neurol. 1993;121:93–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials