Immune responses to West Nile virus infection in the central nervous system - PubMed (original) (raw)
Review
Immune responses to West Nile virus infection in the central nervous system
Hyelim Cho et al. Viruses. 2012.
Abstract
West Nile virus (WNV) continues to cause outbreaks of severe neuroinvasive disease in humans and other vertebrate animals in the United States, Europe, and other regions of the world. This review discusses our understanding of the interactions between virus and host that occur in the central nervous system (CNS), the outcome of which can be protection, viral pathogenesis, or immunopathogenesis. We will focus on defining the current state of knowledge of WNV entry, tropism, and host immune response in the CNS, all of which affect the balance between injury and successful clearance.
Figures
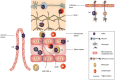
Figure 1
Mechanism of neuroinvasion of West Nile virus (WNV). WNV may enter the central nervous system (CNS) via multiple mechanisms including axonal retrograde transport along peripheral neurons into the spinal cord or hematogenous transport across the blood-brain barrier (BBB). Spinal cord entry is believed to result in interneuron spread to motor neuron cell bodies within the anterior horn of the spinal cord and lead to flaccid paralysis. The possible routes of virus entry across the BBB include (a) “Trojan horse” model; intracellular transport within macrophages or neutrophils, (b) loss of integrity of the BBB; cytokine-mediated (TNF-α, MIF) or matrix metalloproteinases disruption of tight junctions and basement membranes; (c) direct infection of brain microvascular endothelial cells with basolateral spread of the virus; (d) infection of choroid plexus epithelial cells; or (e) direct infection of olfactory neurons adjacent to the cribriform plate.
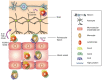
Figure 2
Leukocyte trafficking into the CNS after WNV. Upon WNV infection of neurons, virus-mediated upregulation of Cxcl10 recruits virus-specific CD8+ T cells via interactions with Cxcr3. Expression of Ccl3, Ccl4, and Ccl5 by other neuronal cells recruits Ccr5-expressing leukocytes. Monocytes and lymphocytes entering the perivascular spaces may be retained initially via Cxcr4 binding Cxcl12 [120]. Leukocyte egress from perivascular spaces requires IL-1β, TNF-α, and CD40 interactions, which likely upregulates adhesion molecules including ICAM-1 and VCAM-1 [121,123,124].
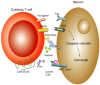
Figure 3
Mechanisms of CD8+ T cell clearance in the CNS. CD8+ T cells control WNV infection in the CNS through multiple mechanisms. Infected neurons upregulate surface expression of MHC class I molecules. Antigen-specific CD8+ T cells recognize infected neurons via class I MHC and processed viral peptides and trigger cell death of target cells through perforin, Fas-Fas ligand, or TRAIL-dependent pathways. Perforin-mediated control of infected neurons occurs through the granzyme-dependent granule exocytosis pathway, which results in apoptosis of infected neuron. Interactions between Fas on infected neurons and FasL on CD8+ T cells leads to programmed cell death of neurons through caspase-dependent pathways. CD8+ T cells also utilize TRAIL to restrict WNV infection in neurons. TRAIL binds to DR5 on neurons, which can have a direct antiviral effect against flaviviruses [125] or result in targeted apoptosis. Activated CD8+ T cell also produce IFN-γ, which can induce genes with antiviral effect.
Similar articles
- The neuroimmune response to West Nile virus.
Fredericksen BL. Fredericksen BL. J Neurovirol. 2014 Apr;20(2):113-21. doi: 10.1007/s13365-013-0180-z. Epub 2013 Jul 11. J Neurovirol. 2014. PMID: 23843081 Free PMC article. Review. - Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus.
Klein RS, Diamond MS. Klein RS, et al. Trends Mol Med. 2008 Jul;14(7):286-94. doi: 10.1016/j.molmed.2008.05.004. Epub 2008 Jun 6. Trends Mol Med. 2008. PMID: 18539532 Review. - West Nile virus: immunity and pathogenesis.
Lim SM, Koraka P, Osterhaus AD, Martina BE. Lim SM, et al. Viruses. 2011 Jun;3(6):811-28. doi: 10.3390/v3060811. Epub 2011 Jun 15. Viruses. 2011. PMID: 21994755 Free PMC article. Review. - Genetic diversity in the collaborative cross model recapitulates human West Nile virus disease outcomes.
Graham JB, Thomas S, Swarts J, McMillan AA, Ferris MT, Suthar MS, Treuting PM, Ireton R, Gale M Jr, Lund JM. Graham JB, et al. mBio. 2015 May 5;6(3):e00493-15. doi: 10.1128/mBio.00493-15. mBio. 2015. PMID: 25944860 Free PMC article. - The host immunologic response to West Nile encephalitis virus.
Diamond MS, Mehlhop E, Oliphant T, Samuel MA. Diamond MS, et al. Front Biosci (Landmark Ed). 2009 Jan 1;14(8):3024-34. doi: 10.2741/3432. Front Biosci (Landmark Ed). 2009. PMID: 19273254 Review.
Cited by
- Virus infections in the nervous system.
Koyuncu OO, Hogue IB, Enquist LW. Koyuncu OO, et al. Cell Host Microbe. 2013 Apr 17;13(4):379-93. doi: 10.1016/j.chom.2013.03.010. Cell Host Microbe. 2013. PMID: 23601101 Free PMC article. Review. - Cross-protection against St. Louis encephalitis virus and Usutu virus by West Nile virus convalescent plasma.
Hossain MS, Vogt MB, Hawks SA, Coutermarsh-Ott SL, Duggal NK. Hossain MS, et al. Virology. 2025 Jul;608:110555. doi: 10.1016/j.virol.2025.110555. Epub 2025 Apr 18. Virology. 2025. PMID: 40273513 - Current developments in understanding of West Nile virus central nervous system disease.
Tyler KL. Tyler KL. Curr Opin Neurol. 2014 Jun;27(3):342-8. doi: 10.1097/WCO.0000000000000088. Curr Opin Neurol. 2014. PMID: 24722324 Free PMC article. Review. - The Japanese Encephalitis Antigenic Complex Viruses: From Structure to Immunity.
Khare B, Kuhn RJ. Khare B, et al. Viruses. 2022 Oct 8;14(10):2213. doi: 10.3390/v14102213. Viruses. 2022. PMID: 36298768 Free PMC article. Review. - Astrocytes Are a Key Target for Neurotropic Viral Infection.
Potokar M, Zorec R, Jorgačevski J. Potokar M, et al. Cells. 2023 Sep 19;12(18):2307. doi: 10.3390/cells12182307. Cells. 2023. PMID: 37759529 Free PMC article. Review.
References
- Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A Neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop Med. Hyg. 1940;1:471–492.
- Kleiboeker S.B. West Nile Virus. In: Nriagu J.O., editor. Encyclopedia of Environmental Health. Elsevier; Burlington, Canada: 2011. pp. 761–768.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources