T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production - PubMed (original) (raw)
T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production
John C Riches et al. Blood. 2013.
Abstract
T-cell exhaustion, originally described in chronic viral infections, was recently reported in solid and hematologic cancers. It is not defined whether exhaustion contributes to T-cell dysfunction observed in chronic lymphocytic leukemia (CLL). We investigated the phenotype and function of T cells from CLL patients and age-matched controls. CD8+ and CD4+ T cells from CLL patients had increased expression of exhaustion markers CD244, CD160, and PD1, with expansion of a PD1+BLIMP1HI subset. These molecules were most highly expressed in the expanded population of effector T cells in CLL. CLL CD8+ T cells showed functional defects in proliferation and cytotoxicity, with the cytolytic defect caused by impaired granzyme packaging into vesicles and nonpolarized degranulation. In contrast to virally induced exhaustion, CLL T cells showed increased production of interferon-γ and TNFα and increased expression of TBET, and normal IL2 production. These defects were not restricted to expanded populations of cytomegalovirus (CMV)–specific cells, although CMV seropositivity modulated the distribution of lymphocyte subsets, the functional defects were present irrespective of CMV serostatus. Therefore, although CLL CD8+ T cells exhibit features of T-cell exhaustion, they retain the ability to produce cytokines. These findings also exclude CMV as the sole cause of T-cell defects in CLL.
Figures
Figure 1
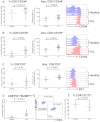
Increased expression of PD1, CD160, and CD244 on CD8+ T cells from patients with CLL. The expression of (A) CD244, (B) CD160, and (C) PD1 were assessed on CD8+ T cells from patients with CLL and healthy age-matched controls. Significant increases in both the proportion, and the absolute number, of T cells expressing these molecules were observed. (D) PD1 expression identified a population of CD3+CD8+ T cells with high expression of BLIMP1, which are expanded in CLL. (E) Flow cytometric plots showing the presence of this CD3+CD8+PD1+BLIMP1HI population that is increased in CLL patients. (F) Expression of CD127 is not significantly down-regulated on CLL T cells.
Figure 2
CD244, CD160, and PD1 are preferentially expressed on CD3+CD8+CCR7− effector T cells which are increased in CLL. (A) CLL patients had an increased proportion (A) and increased numbers (B) of CD3+CD8+CCR7− effector T cells. The relative expression of CD160, CD244, and PD1 was analyzed on CD8+ lymphocyte subsets from CLL patients as defined by coexpression of CCR7 and CD45RA. Quadrant gates were used to compare the MFI of CD160, CD244, and PD1 on CCR7+CD45RA+ naive cells, CCR7+CD45RA− TCM cells, CCR7−CD45RA− TEM cells, and CCR7−CD45RA+ TEMRA cells. Expression of CD244 (C) and CD160 (D) was increased on TEM cells, and was highest on TEMRA cells. (E) Expression of CD244 was virtually specific for a CCR7− phenotype. (F) In contrast, expression of PD1 was highest on TEM cells.
Figure 3
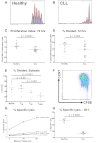
CD8+ T cells from patients with CLL show functional defects in proliferation and cytoxicity. The proliferative potential of CD8+ T cells from CLL patients and healthy controls was assessed using a CFSE based assay. Representative histograms are shown of (A) a healthy donor and (B) a CLL patient. (C) The proliferation index was significantly lower for CD8+ T cells from CLL patients compared with healthy controls. (D) There was also a reduction in the proportion of CD8+ T cells that had entered cell division (percentage divided) in CLL patients compared with healthy controls. The proliferative capacity of the various CD8+ lymphocyte subsets as defined by coexpression of CCR7 and CD45RA was assessed. (E-F) CD8+CCR7− T cells (TEM and TEMRA) had reduced proliferative capacity compared with CD8+CCR7− T cells (Naive and TCM). The cytotoxic activity of CD8+ T cells from patients and controls was assessed. CD8+ T cells from CLL patients fail to lyse idiotype-pulsed target cells in contrast to CD8+ T cells from healthy controls: percentage specific lysis at all effector target ratios (G), and at a effector-target ratio of 30:1 (H).
Figure 4
CD8+ T cells from CLL patients show increased production of IFNγ and TNFα, and increased expression of TBET, but normal production of IL2. Cytokine production by CD8+ T cells was assessed by intracytoplasmic staining for IFNγ, TNFα, and IL2 after stimulation with PMA and ionomycin. CD8+ T cells from CLL patients showed increased production of IFNγ (A) and TNFα (B) reflecting the global skew toward CD8+CCR7− subsets (TEM and TEMRA). (C) In contrast, there was no significant difference in the production of IL2 by CD8+ T cells from patients and controls. (D) CD160 was expressed on CD8+ T cells that produced IFNγ but not on cells producing IL4, consistent with its expression being restricted to Tc1 cells. (E) The expression of TBET in CD8+ T-cell subsets from CLL patients was analyzed as defined by coexpression of CCR7 and CD45RA as before. The expression of TBET was increased in TEM cells, with the highest expression levels in the TEMRA subset. (F) CD3+CD8+CCR7− T cells from patients with CLL show significantly increased TBET expression compared with controls.
Figure 5
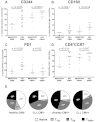
The impact of CMV serostatus on the distribution of subsets and expression of CD244, CD160, and PD1. The proportion of T cells expressing PD1, CD160, and CD244 were measured by flow cytometry in patients with CLL and healthy controls matched for CMV serostatus. Significant increases in the expression of CD244 (A) and CD160 (B) were observed independently of CMV serostatus. (C) In contrast, expression of PD1 was only increased comparing CMV− patients and controls. (D) CD3+CD8+CCR7− cells are expanded in CLL irrespective of CMV serostatus. (E) There is a reduction in naive and central memory cells because of both CLL and CMV seropositivity. The presence of CLL skews the T-cell repertoire toward a TEM phenotype, whereas the presence of CMV leads to an expansion of TEMRA cells.
Figure 6
The defects in T-cell function seen in CLL are present irrespective of CMV serostatus. The function of CLL CD8+ T cells was compared with CD8+ T cells from healthy controls matched for CMV serostatus. Increased production of IFNγ (A), increased expression of TBET (B), decreased proliferative capacity (C), and decreased cytolytic ability (D) of CLL T cells were all found irrespective of CMV serostatus. Pulsed target cells (closed symbols); unpulsed control target cells (open symbols). The graph representing percentage-specific lysis shows the mean and standard error of results obtained from 13 CLL patients, (5 CMV− and 8 CMV+), and 12 healthy controls (5 CMV− and 7 CMV+).
Figure 7
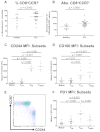
CD8+ T cells from CLL patients show defective cytotoxicity because of failure of granzyme localization to the immunologic synapse. The cytotoxic activity of CD8+ T cells from CLL patients was assessed further. (A) CD8+ T cells from CLL patients retain the ability to degranulate in response to SEB, as shown by their increased ability to transfer CD107a to the cell surface. (B) Healthy CD8+ T cells showed effective F-actin (red, rhodamine phalloidin) immunologic synapse formation with colocalization of CD107a (yellow, Alexa Fluor 647) and granzyme B (green, Alexa Fluor 488) at the synapse contact site with healthy B cells (+sAg). (C) In contrast, CD8+ T cells from CLL patients fail to form effective F-actin immune synapses with autologous CLL cells (+sAg) that is associated with strong, nonpolarized expression of CD107a and a lack of granzyme B polarization to the contact site.
Comment in
- Exhausting T cells in CLL.
Zenz T. Zenz T. Blood. 2013 Feb 28;121(9):1485-6. doi: 10.1182/blood-2013-01-475939. Blood. 2013. PMID: 23449612
Similar articles
- Expanded antigen-experienced CD160+CD8+effector T cells exhibit impaired effector functions in chronic lymphocytic leukemia.
Bozorgmehr N, Okoye I, Oyegbami O, Xu L, Fontaine A, Cox-Kennett N, Larratt LM, Hnatiuk M, Fagarasanu A, Brandwein J, Peters AC, Elahi S. Bozorgmehr N, et al. J Immunother Cancer. 2021 Apr;9(4):e002189. doi: 10.1136/jitc-2020-002189. J Immunother Cancer. 2021. PMID: 33931471 Free PMC article. - CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukemia.
te Raa GD, Pascutti MF, García-Vallejo JJ, Reinen E, Remmerswaal EB, ten Berge IJ, van Lier RA, Eldering E, van Oers MH, Tonino SH, Kater AP. te Raa GD, et al. Blood. 2014 Jan 30;123(5):717-24. doi: 10.1182/blood-2013-08-518183. Epub 2013 Nov 18. Blood. 2014. PMID: 24246502 - Expansion of effector T cells associated with decreased PD-1 expression in patients with indolent B cell lymphomas and chronic lymphocytic leukemia.
Tonino SH, van de Berg PJ, Yong SL, ten Berge IJ, Kersten MJ, van Lier RA, van Oers MH, Kater AP. Tonino SH, et al. Leuk Lymphoma. 2012 Sep;53(9):1785-94. doi: 10.3109/10428194.2012.673224. Epub 2012 Apr 19. Leuk Lymphoma. 2012. PMID: 22397719 - Abnormal T-cell function in B-cell chronic lymphocytic leukaemia.
Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Scrivener S, et al. Leuk Lymphoma. 2003 Mar;44(3):383-9. doi: 10.1080/1042819021000029993. Leuk Lymphoma. 2003. PMID: 12688308 Review. - CD160 receptor in CLL: Current state and future avenues.
Oumeslakht L, Aziz AI, Bensussan A, Ben Mkaddem S. Oumeslakht L, et al. Front Immunol. 2022 Nov 7;13:1028013. doi: 10.3389/fimmu.2022.1028013. eCollection 2022. Front Immunol. 2022. PMID: 36420268 Free PMC article. Review.
Cited by
- Causes of death among patients diagnosed with chronic lymphocytic leukemia: A population-based study in the Netherlands, 1996-2020.
van der Straten L, Levin MD, Dinnessen MAW, Visser O, Posthuma EFM, Doorduijn JK, Langerak AW, Kater AP, Dinmohamed AG. van der Straten L, et al. Hemasphere. 2024 Nov 12;8(11):e70015. doi: 10.1002/hem3.70015. eCollection 2024 Nov. Hemasphere. 2024. PMID: 39534384 Free PMC article. - Assaying and classifying T cell function by cell morphology.
Wang X, Fernandes SM, Brown JR, Kam LC. Wang X, et al. BioMedInformatics. 2024 Jun;4(2):1144-1154. doi: 10.3390/biomedinformatics4020063. Epub 2024 Apr 26. BioMedInformatics. 2024. PMID: 39525274 - The Immunomodulatory Mechanisms of BTK Inhibition in CLL and Beyond.
Jiang Q, Peng Y, Herling CD, Herling M. Jiang Q, et al. Cancers (Basel). 2024 Oct 23;16(21):3574. doi: 10.3390/cancers16213574. Cancers (Basel). 2024. PMID: 39518015 Free PMC article. Review. - Profound deficiencies in mature blood and bone marrow progenitor dendritic cells in Chronic Lymphocyticcytic Leukemia patients.
Welch BM, Parikh SA, Kay NE, Medina KL. Welch BM, et al. Res Sq [Preprint]. 2024 Sep 27:rs.3.rs-4953853. doi: 10.21203/rs.3.rs-4953853/v1. Res Sq. 2024. PMID: 39399662 Free PMC article. Preprint. - Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib.
Stéphan P, Bouherrou K, Guillermin Y, Michallet AS, Grinberg-Bleyer Y. Stéphan P, et al. Cells. 2024 Aug 30;13(17):1458. doi: 10.3390/cells13171458. Cells. 2024. PMID: 39273028 Free PMC article.
References
- Hofbauer JP, Heyder C, Denk U, et al. Development of CLL in the TCL1 transgenic mouse model is associated with severe skewing of the T-cell compartment homologous to human CLL. Leukemia. 2011;25(9):1452–1458. - PubMed
- Catovsky D, Miliani E, Okos A, Galton DA. Clinical significance of T-cells in chronic lymphocytic leukaemia. Lancet. 1974;2(7883):751–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0902269/MRC_/Medical Research Council/United Kingdom
- P01 CA081534/CA/NCI NIH HHS/United States
- P01 CA095426/CA/NCI NIH HHS/United States
- P01 CA95426/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials