Inducible endothelium-derived hyperpolarizing factor: role of the 15-lipoxygenase-EDHF pathway - PubMed (original) (raw)
Review
Inducible endothelium-derived hyperpolarizing factor: role of the 15-lipoxygenase-EDHF pathway
William B Campbell et al. J Cardiovasc Pharmacol. 2013 Mar.
Abstract
Endothelium-derived hyperpolarizing factors (EDHFs) regulate vascular tone by contributing to the vasorelaxations to shear stress and endothelial agonists such as bradykinin and acetylcholine. 15(S)-Hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA) and 11(R),12(S),15(S)-trihydroxyeicosatrienoic acid (11,12,15-THETA) are endothelial metabolites of the 15-lipoxygenase (15-LO) pathway of arachidonic acid metabolism and are EDHFs. 11,12,15-THETA activates small conductance, calcium-activated potassium channels on smooth muscle cells causing membrane hyperpolarization, and relaxation. Expression levels of 15-LO in the endothelium regulate the activity of the 15-LO/15-H-11,12-EETA/11,12,15-THETA pathway and its contribution to vascular tone. Regulation of its expression is by transcriptional, translational, and epigenetic mechanisms. Hypoxia, hypercholesterolemia, atherosclerosis, anemia, estrogen, interleukins, and possibly other hormones increase 15-LO expression. An increase in 15-LO results in increased synthesis of 15-H-11,12-EETA and 11,12,15-THETA, increased membrane hyperpolarization, and enhanced contribution to relaxation by endothelial agonists. Thus, the 15-LO pathway represents the first example of an inducible EDHF. In addition to 15-LO metabolites, a number of chemicals have been identified as EDHFs and their contributions to vascular tone vary with species and vascular bed. The reason for multiple EDHFs has evaded explanation. However, EDHF functioning as constitutive EDHFs or inducible EDHFs may explain the need for chemically and biochemically distinct pathways for EDHF activity and the variation in EDHFs between species and vascular beds. This new EDHF classification provides a framework for understanding EDHF activity in physiological and pathological conditions.
Figures
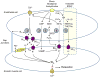
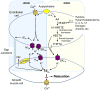
Figure 1
Signaling mechanisms of the five major endothelium-derived hyperpolarizing factors (EDHFs). Shear stress or endothelium-dependent agonists including acetylcholine and bradykinin stimulate EDHF-dependent vascular relaxation. EDHF mediators include: (1) electrical transmission of endothelial hyperpolarization through myoendothelial gap junctions, (1) K ion, (2) C-type natriuretic peptide (CNP), (3) hydrogen peroxide (H2O2), (4) epoxyeicosatrienoic acids (EETs) and (5) 15-lipoxygenase-1 (15-LO-1) metabolites, 15-H-11,12-EETA and 11,12,15-THETA. The 15-LO-1 inducible EDHF pathway is highlighted. The shaded numbers in the figure refer to the Classification of EDHF section of the text and correspond to numbered descriptions of 5 major EDHFs.
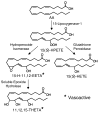
Figure 2
15-Lipoxygenase-1 pathway of endothelial cell arachidonic acid (AA) metabolism. 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HPETE), 15-hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA), 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA). * 15-H-11,12-EETA and 11,12,15-THETA cause vascular relaxation and function as EDHFs.
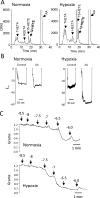
Figure 3
Effect of hypoxia on 15-LO activity in the rabbit vasculature. (A) AA metabolism of rabbit aortic endothelial cells exposed to normal oxygen (21% O2, normoxia) or reduced oxygen (0.7% O2, hypoxia). Endothelial cells were incubated with 14C-AA in the presence of indomethacin (10 μM) for 8 h under normoxic or hypoxic conditions. The media was removed, extracted and metabolites resolved by reverse-phase HPLC. Radioactivity of column fractions was measured by scintillation counting. Migration times of known standards are noted on each chromatogram. (B) AA-induced hyperpolarization responses in mesenteric arteries from normoxic or hypoxic rabbits. Male 8 week old rabbits were exposed to either normoxic conditions (21% O2) or hypoxic conditions (12% O2) for 5 days. Membrane potential cell impalement recordings were made in the freshly dissected arterial segments incubated with indomethacin (10 μM) and phenylephrine (100 nM) with or without AA (10 μM). (C) Acetylcholine relaxations of mesenteric arteries from normoxic (top trace) or hypoxic (bottom trace) rabbits. Arterial rings were mounted in a myograph, stretched to a basal tension of 1 gram, treated with indomethacin (10 μM) and N-nitro-L-arginine (30 μM) and constricted with phenylephrine (0.1 – 1.0 μM). Increasing concentrations of acetylcholine were added and relaxation responses recorded.
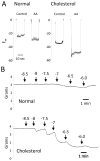
Figure 4
Effect of a high cholesterol diet on aortic vascular activity. Male 8 week old rabbits were fed either normal chow (normal) or cholesterol enriched (2% cholesterol) chow (cholesterol) for 2 weeks. (A) AA-induced hyperpolarization responses. Membrane potential cell impalement recordings were made in the freshly dissected aortic segments incubated with indomethacin (10 μM) and phenylephrine (100 nM) with or without AA (10 μM). (B) Acetylcholine relaxations. Arterial rings from rabbits fed normal chow (top trace) or rabbits fed cholesterol-enriched chow (bottom trace) were mounted in a myograph, stretched to a basal tension of 2 grams, treated with indomethacin (10 μM) and N-nitro-L-arginine (30 μM) and constricted with phenylephrine (0.1 – 1.0 μM). Increasing concentrations of acetylcholine were added and relaxation responses recorded.
Figure 5
Co-release of constitutive (c) and inducible (i) EDHFs mediate synergistic vasorelaxation. In vascular endothelial cells, acetylcholine activates; 1) calcium influx which stimulates IKCa and SKCa channels resulting in K ion efflux (the cEDHF pathway) and 2) PLA2 release of AA from membrane phospholipids. AA is metabolized by 15-LO-1 to HEETA and THETA (the iEDHF pathway). 15-LO-1 expression is increased by hypoxia, hypercholesterolemia, interleukin-4 (IL-4), interleukin-13 (IL-13), estrogen and anemia. The cEDHF and iEDHF pathways cause smooth muscle hyperpolarization via distinct synergistic mechanisms. For the cEDHF pathway, endothelial cell hyperpolarization from K ion efflux is transmitted to the smooth muscle through myoendothelial gap junctions or K ions activate smooth muscle KIR channels and the Na/K ATPase. HEETAs and THEETAs from the iEDHF pathway activate smooth muscle SKCa channels.
Similar articles
- Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone.
Chawengsub Y, Gauthier KM, Campbell WB. Chawengsub Y, et al. Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H495-507. doi: 10.1152/ajpheart.00349.2009. Epub 2009 Jun 12. Am J Physiol Heart Circ Physiol. 2009. PMID: 19525377 Free PMC article. Review. - Effect of human 15-lipoxygenase-1 metabolites on vascular function in mouse mesenteric arteries and hearts.
Kriska T, Cepura C, Siangjong L, Wan TC, Auchampach JA, Shaish A, Haratz D, Kumar G, Falck JR, Gauthier KM, Campbell WB. Kriska T, et al. Prostaglandins Other Lipid Mediat. 2013 Oct;106:8-15. doi: 10.1016/j.prostaglandins.2013.07.002. Epub 2013 Jul 16. Prostaglandins Other Lipid Mediat. 2013. PMID: 23872364 Free PMC article. - Chronic hypoxia enhances 15-lipoxygenase-mediated vasorelaxation in rabbit arteries.
Aggarwal NT, Pfister SL, Gauthier KM, Chawengsub Y, Baker JE, Campbell WB. Aggarwal NT, et al. Am J Physiol Heart Circ Physiol. 2009 Mar;296(3):H678-88. doi: 10.1152/ajpheart.00777.2008. Epub 2008 Dec 26. Am J Physiol Heart Circ Physiol. 2009. PMID: 19112096 Free PMC article. Retracted. - A transferable, beta-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells.
Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. Popp R, et al. J Physiol. 1996 Dec 15;497 ( Pt 3)(Pt 3):699-709. doi: 10.1113/jphysiol.1996.sp021801. J Physiol. 1996. PMID: 9003555 Free PMC article. - Endothelium-derived hyperpolarizing factor and endothelium-dependent relaxations.
Nagao T, Vanhoutte PM. Nagao T, et al. Am J Respir Cell Mol Biol. 1993 Jan;8(1):1-6. doi: 10.1165/ajrcmb/8.1.1. Am J Respir Cell Mol Biol. 1993. PMID: 8380248 Review.
Cited by
- The Gatekeepers in the Mouse Ophthalmic Artery: Endothelium-Dependent Mechanisms of Cholinergic Vasodilation.
Manicam C, Staubitz J, Brochhausen C, Grus FH, Pfeiffer N, Gericke A. Manicam C, et al. Sci Rep. 2016 Feb 2;6:20322. doi: 10.1038/srep20322. Sci Rep. 2016. PMID: 26831940 Free PMC article. - Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation.
Bekassy Z, Lopatko Fagerström I, Bader M, Karpman D. Bekassy Z, et al. Nat Rev Immunol. 2022 Jul;22(7):411-428. doi: 10.1038/s41577-021-00634-8. Epub 2021 Nov 10. Nat Rev Immunol. 2022. PMID: 34759348 Free PMC article. Review. - Vascular Reactivity Profile of Novel KCa 3.1-Selective Positive-Gating Modulators in the Coronary Vascular Bed.
Oliván-Viguera A, Valero MS, Pinilla E, Amor S, García-Villalón ÁL, Coleman N, Laría C, Calvín-Tienza V, García-Otín ÁL, Fernández-Fernández JM, Murillo MD, Gálvez JA, Díaz-de-Villegas MD, Badorrey R, Simonsen U, Rivera L, Wulff H, Köhler R. Oliván-Viguera A, et al. Basic Clin Pharmacol Toxicol. 2016 Aug;119(2):184-92. doi: 10.1111/bcpt.12560. Epub 2016 Feb 29. Basic Clin Pharmacol Toxicol. 2016. PMID: 26821335 Free PMC article. - The Vascular Effects of Isolated Isoflavones-A Focus on the Determinants of Blood Pressure Regulation.
Silva H. Silva H. Biology (Basel). 2021 Jan 12;10(1):49. doi: 10.3390/biology10010049. Biology (Basel). 2021. PMID: 33445531 Free PMC article. Review.
References
- Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. - PubMed
- Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: Beyond nitric oxide and cyclic gmp. Circulation. 1995;92:3337–3349. - PubMed
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolaizing factor. Where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. - PubMed
- Nagao T, Illiano S, Vanhoutte PM. Heterogenous distribution of endothelium-dependent relaxations resistant to n-nitro-l-arginine in rats. Am J Physiol. 1992;263:H1090–H1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources