Autophagy and apoptosis are differentially induced in neurons and astrocytes treated with an in vitro mimic of the ischemic penumbra - PubMed (original) (raw)
Autophagy and apoptosis are differentially induced in neurons and astrocytes treated with an in vitro mimic of the ischemic penumbra
Matthew E Pamenter et al. PLoS One. 2012.
Abstract
The development of clinical stroke therapies remains elusive. The neuroprotective efficacies of thousands of molecules and compounds have not yet been determined; however, screening large volumes of potential targets in vivo is severely rate limiting. High throughput screens (HTS) may be used to discover promising candidates, but this approach has been hindered by the lack of a simple in vitro model of the ischemic penumbra, a clinically relevant region of stroke-afflicted brain. Recently, our laboratory developed such a mimic (ischemic solution: IS) suitable for HTS, but the etiology of stress pathways activated by this model are poorly understood. The aim of the present study was to determine if the cell death phenotype induced by IS accurately mimics the in vivo penumbra and thus whether our model system is suitable for use in HTS. We treated cultured neuron and astrocyte cell lines with IS for up to 48 hrs and examined cellular energy state ([ATP]), cell and organelle morphology, and gene and molecular profiles related to stress pathways. We found that IS-treated cells exhibited a phenotype of mixed apoptosis/autophagy characteristic of the in vivo penumbra, including: (1) short-term elevation of [ATP] followed by progressive ATP depletion and Poly ADP Ribose Polymerase cleavage, (2) increased vacuole number in the cytoplasm, (3) mitochondrial rupture, decreased mitochondrial and cristae density, release of cytochrome C and apoptosis inducing factor, (4) chromatin condensation, nuclear lamin A and DNA cleavage, fragmentation of the nuclear envelope, and (5) altered expression of mRNA and proteins consistent with autophagy and apoptosis. We conclude that our in vitro model of the ischemic penumbra induces autophagy and apoptosis in cultured neuron and astrocyte cell lines and that this mimic solution is suitable for use in HTS to elucidate neuroprotective candidates against ischemic penumbral cell death.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
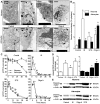
Figure 1. IS induces extensive autophagic vacuolization of neuronal cytoplasm and depletes [ATP] in neurons and astrocytes.
IS-treated neurons and oligomycin-treated neurons and astrocytes exhibit extensive cytoplasmic vacuolization, organelle digestion, and [ATP] changes characteristic of autophagy. (A) Sample TEM images of neurons (upper panels) and astrocytes (lower panels) treated as indicated for 24 hrs. Arrows indicate vacuoles. (B) Summary of vacuole density in the cytoplasm by volume. TEM experiments were repeated 2–3 times and 10–20 cells were examined from each treatment group. (C) Summary of change in [ATP] with time from neurons (open symbols) and astrocytes (closed symbols) treated as indicated through 48 hrs from >10 different experiments. (D) Summary of fold-change in protein expression from western blot analysis of PARP cleavage in samples treated for 6 hrs. Changes were normalized to α–actin expression in the same sample. (E) Sample western blots from (D). Blots are representative of 3 separate experiments. Data are mean ± SEM. Asterisks (*) indicate significant difference from untreated controls (p<0.05). Treatments: control (DMEM/F12), ischemic solution (IS), 2.5 µm staurosporine (STS), and 10 µm oligomycin A (Oligo A).
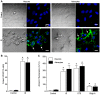
Figure 2. IS induces apoptotic Annexin V translocation in neurons and astrocytes.
(A) Sample paired DIC (left panels) and Annexin V and DAPI (green and blue fluorescence, respectively; right panels) confocal microscopy images of neurons and astrocytes treated as indicated for 24 hrs. Images are representative of 4 separate experiments. (B) Summary of the ratio of Annexin V-positive stained cells to DAPI-stained nuclei. (C) Summary of Annexin V fluorescence from neurons or astrocytes treated in 96-well microplates as indicated for 24 hrs. Data are mean ± SEM. Asterisks (*) indicated significant difference from untreated controls (p<0.05).
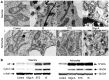
Figure 3. IS causes mitochondrial fission and membrane rupture.
(A) Sample TEM images of mitochondria from neurons (upper) and astrocytes (lower) treated as indicated for 24 hrs. (B) Sample western blots of apoptosis inducing factor (AIF) and cytochrome C (Cyto C) release from samples treated as indicated for 6 hrs. Blots are representative of 3 separate experiments.
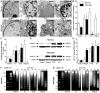
Figure 4. IS-treated nuclei exhibit apoptotic chromatin condensation, nuclear envelope fragmentation into apoptotic bodies, and laddered DNA cleavage.
(A) Sample TEM images of nuclei from neurons (upper panels) and astrocytes (lower panels) treated as indicated for 24 hrs. White arrows indicate condensed chromatin beads and fragmenting nuclei. (B) Summary of nuclear volume density from samples in (A). (C) Summary of chromatin volume density from samples in (A). Note: nuclei were too fragmented in oligomycin A-treated astrocytes for quantification. (D) Sample western blots of lamin A cleavage in samples treated as indicated for 6 hrs. Blots are representative of 3 separate experiments. (E) Summary of fold-change in protein expression from (D) normalized to α–actin expression in the same sample. (F&G) Sample conventional agarose gel electrophoresis gel images of DNA fragmentation from neurons (F) and astrocytes (G) treated as indicated for 0, 12, 24, or 48 hrs. Gels are representative of 4 separate experiments. Data are mean ± SEM. Asterisks (*) indicate significant difference from untreated controls (p<0.05).
Figure 5. IS upregulates autophagy and apoptotic pathways in neurons and astrocytes.
(A) Sample Western blots of autophagy- and apoptosis related protein expression from neurons (left panels) and astrocytes (right panels) treated as indicated for 6 hrs. (B) Summary of fold-change in protein expressions from (A) normalized to α–actin expression in the same sample. Data are mean ± SEM from 3 separate experiments for each protein, cell type, and treatment. Asterisks (*) indicate significant difference from untreated controls (p<0.05).
Similar articles
- Ischemia-induced apoptosis in primary cultures of astrocytes.
Yu AC, Wong HK, Yung HW, Lau LT. Yu AC, et al. Glia. 2001 Aug;35(2):121-30. doi: 10.1002/glia.1077. Glia. 2001. PMID: 11460268 - Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke.
Pei X, Li Y, Zhu L, Zhou Z. Pei X, et al. Exp Cell Res. 2019 Sep 15;382(2):111474. doi: 10.1016/j.yexcr.2019.06.019. Epub 2019 Jun 21. Exp Cell Res. 2019. PMID: 31229506 - DIDS (4,4-diisothiocyanatostilbenedisulphonic acid) induces apoptotic cell death in a hippocampal neuronal cell line and is not neuroprotective against ischemic stress.
Pamenter ME, Perkins GA, Gu XQ, Ellisman MH, Haddad GG. Pamenter ME, et al. PLoS One. 2013;8(4):e60804. doi: 10.1371/journal.pone.0060804. Epub 2013 Apr 5. PLoS One. 2013. PMID: 23577164 Free PMC article. - Apoptosis and Acute Brain Ischemia in Ischemic Stroke.
Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER. Radak D, et al. Curr Vasc Pharmacol. 2017;15(2):115-122. doi: 10.2174/1570161115666161104095522. Curr Vasc Pharmacol. 2017. PMID: 27823556 Review. - Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection.
Bambrick L, Kristian T, Fiskum G. Bambrick L, et al. Neurochem Res. 2004 Mar;29(3):601-8. doi: 10.1023/b:nere.0000014830.06376.e6. Neurochem Res. 2004. PMID: 15038607 Review.
Cited by
- Sphingosine kinase 1-associated autophagy differs between neurons and astrocytes.
Moruno-Manchon JF, Uzor NE, Ambati CR, Shetty V, Putluri N, Jagannath C, McCullough LD, Tsvetkov AS. Moruno-Manchon JF, et al. Cell Death Dis. 2018 May 1;9(5):521. doi: 10.1038/s41419-018-0599-5. Cell Death Dis. 2018. PMID: 29743513 Free PMC article. - The Contribution of Astrocyte Autophagy to Systemic Metabolism.
Ortiz-Rodriguez A, Arevalo MA. Ortiz-Rodriguez A, et al. Int J Mol Sci. 2020 Apr 3;21(7):2479. doi: 10.3390/ijms21072479. Int J Mol Sci. 2020. PMID: 32260050 Free PMC article. Review. - Peroxisomal Dysfunction in Neurological Diseases and Brain Aging.
Uzor NE, McCullough LD, Tsvetkov AS. Uzor NE, et al. Front Cell Neurosci. 2020 Mar 10;14:44. doi: 10.3389/fncel.2020.00044. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32210766 Free PMC article. - Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes.
Zhou XY, Luo Y, Zhu YM, Liu ZH, Kent TA, Rong JG, Li W, Qiao SG, Li M, Ni Y, Ishidoh K, Zhang HL. Zhou XY, et al. Cell Death Dis. 2017 Feb 16;8(2):e2618. doi: 10.1038/cddis.2017.34. Cell Death Dis. 2017. PMID: 28206988 Free PMC article. - Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke.
Deng YH, He HY, Yang LQ, Zhang PY. Deng YH, et al. Neural Regen Res. 2016 Jul;11(7):1108-14. doi: 10.4103/1673-5374.187045. Neural Regen Res. 2016. PMID: 27630694 Free PMC article.
References
- Branston NM, Symon L, Crockard HA, Pasztor E (1974) Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol 45: 195–208. - PubMed
- Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA (1983) Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke 14: 332–341. - PubMed
- Lo EH (2008) A new penumbra: transitioning from injury into repair after stroke. Nat Med 14: 497–500. - PubMed
- Candelario-Jalil E (2009) Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs 10: 644–654. - PubMed
- Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40: e331–339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources