Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population - PubMed (original) (raw)
Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population
Luis Arnes et al. PLoS One. 2012.
Abstract
Pancreatic islet cells provide the major source of counteractive endocrine hormones required for maintaining glucose homeostasis; severe health problems result when these cell types are insufficiently active or reduced in number. Therefore, the process of islet endocrine cell lineage allocation is critical to ensure there is a correct balance of islet cell types. There are four endocrine cell types within the adult islet, including the glucagon-producing alpha cells, insulin-producing beta cells, somatostatin-producing delta cells and pancreatic polypeptide-producing PP cells. A fifth islet cell type, the ghrelin-producing epsilon cells, is primarily found during gestational development. Although hormone expression is generally assumed to mark the final entry to a determined cell state, we demonstrate in this study that ghrelin-expressing epsilon cells within the mouse pancreas do not represent a terminally differentiated endocrine population. Ghrelin cells give rise to significant numbers of alpha and PP cells and rare beta cells in the adult islet. Furthermore, pancreatic ghrelin-producing cells are maintained in pancreata lacking the essential endocrine lineage regulator Neurogenin3, and retain the ability to contribute to cells within the pancreatic ductal and exocrine lineages. These results demonstrate that the islet ghrelin-expressing epsilon cells represent a multi-potent progenitor cell population that delineates a major subgrouping of the islet endocrine cell populations. These studies also provide evidence that many of hormone-producing cells within the adult islet represent heterogeneous populations based on their ontogeny, which could have broader implications on the regulation of islet cell ratios and their ability to effectively respond to fluctuations in the metabolic environment during development.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
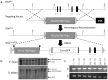
Figure 1. Construction of the GhrlLCA acceptor and Ghrl:Cre-eGFP exchange alleles.
(a) Schematic of the Ghrl:Cre-eGFP knock-in allele generated using recombinase mediated cassette exchange (RMCE). First, a floxed Ghrelin (GhrlLCA) allele was created by inserting a positive/negative selection cassette flanked by the Lox71 and Lox2272 modified lox sites into the ghrelin locus by homologous recombination. The Lox71 and Lox2272 sites allow for directional and irreversible Cre mediated recombination while suppressing intramolecular recombination. An exchange cassette vector containing the complimentary Lox66 and Lox2272 sites and a Cre-eGFP coding sequence spanning exons 2 and 3 (Ghrl:Cre-eGFP) was then created and inserted into the genome using RMCE. (b) Insertion of the GhrlLCA allele was confirmed by Southern blot using either ScaI digested (5′ recombination) or NdeI digested (3′ recombination) genomic DNA. (c) Correct exchange of the Ghrl:Cre-eGFP allele in clones that survived both positive and negative selection was confirmed by PCR analysis. All seven tested clones had correct allele exchange.
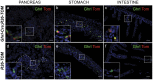
Figure 2. Reporter expression accurately recapitulates ghrelin expression at e18.5.
Tom+ cells co-express ghrelin in pancreas (a), stomach (b) and duodenum (c). Note that the pancreatic ghrelin-producing epsilon population only represent 5–10% of the total pancreatic endocrine population at this stage of development. No leakiness of the reporter was detected in the absence of the Ghrl:Cre allele in any of the tissues studied (d–f). Scale bar, all panels 50 µm, insets 20 µm.
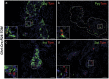
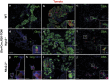
Figure 3. Ghrelin-labeled cells mark a subpopulation of glucagon- and Ppy-expressing cells in neonates.
Representative images showing immunofluorescence of islet hormones and direct tomato reporter fluorescence on cryosections of Ghrl-Cre;R26-Tomato (TOM) P0 pancreas (a-d). Reporter fluorescence co-localized with a significant number of glucagon-expressing cells (a) and Ppy-expressing cells (b). No significant co-localization was detected with insulin- (c) and somatostatin-expressing cells (d). DAPI staining was used to visualize the nuclei. Scale bars: low magnification = 50 µm, insets = 10 µm.
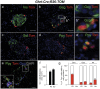
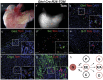
Figure 4. Two distinct populations of alpha and PP cells can be distinguished in the adult endocrine pancreas based on their origin.
We analyzed pancreata from 8 week old (8w) Ghrl-Cre;R26-TOM mice. In the adult, we could not detect ghrelin expression by immunofluorescence but ghrelin-lineage labeled cells localized primarily to the periphery of the islet. (a–b) Adjacent images of the same islet of Langerhans showing co-localization of the ghrelin-lineage labeled cells with glucagon (b) but not with insulin (a). White boxes in b shows two distinct population of alpha cells, one derived from a ghrelin-expressing cell (magnified in b”) and one independent of the ghrelin lineage (magnified in b). (c–d) Adjacent images show that ghrelin-expressing cells contribute to the PP (magnified in d’) but not to the delta lineage. (e) Immunofluorescence analysis with glucagon and Ppy antibodies confirm that two independent populations of alpha and PP cells are derived from ghrelin-expressing cells. (f) Quantitative analysis of the total number of ghrelin-labeled cells per endocrine area at the two stages studied, P0 and 8w pancreas. (g) Fraction of ghrelin-labeled cells within a given population of hormone producing cells at P0 and 8w. The percentage of ghrelin cells labeled with the reporter at P0 is indicative of the Cre recombination efficiency. No ghrelin cells were detected at 8w. Three mice were examined per time point. Error bars show mean ± SEM (p<0.05 (*) Student’s unpaired t-test). DAPI staining was used to visualize the nuclei. Scale bar: low magnification = 50 µm, B, B’ and inset in E = 10 µm, d’ = 20 µm.
Figure 5. Ghrelin lineage-labeled cells are frequently found in the pancreatic exocrine and ductal compartments in neonates.
Ghrelin-lineage labeled Tom+ cells predominantly localize to the islet periphery (a). Approximately 50% of the embryos analyzed contained ghrelin-labeled cells within the amylase+ acini clusters (b). This phenomenon is preferentially observed in juxta-duodenal regions of the ventral pancreas. (c) Ghrelin-lineage labeled cells that co-expressed ductal markers were also identified in the same animals. In Neurog3−/− pancreata (d–f), which are almost completely devoid of endocrine hormone-producing cells, we could detect a small number of ghrelin-expressing and lineage labeled cells (d). We also detected ghrelin descendants in the exocrine (e) and ductal tissue (f) of Neurog3−/− embryos. (g–j) In Nkx2.2−/− mice, a subset of Ppy- (g) and glucagon-expressing cells (h) are still derived from ghrelin-expressing cells. Ghrelin-labeled cells are also detected in the exocrine and ductal compartment of Nkx2.2−/− animals (i–j). The frequency and magnitude of lineage labeling of these cells were not significantly different from wild type pancreas. a, d and e are representative images of at least 3 pancreata. b, c, f, g, h, i, j are representative image of at least 3 embryos in which we detected labeled cells localized to the exocrine and ductal pancreatic tissue. All images are from Ghrl-Cre;R26-TOM P0 pancreas in the indicated genetic backgrounds. DAPI staining was used to visualize the nuclei. Scale bar: all panels = 50 µm, insets = 10 µm.
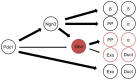
Figure 6. Ghrelin lineage-labeled cells are committed to the endocrine lineage and do not differentiate into chief nor parietal cells in the stomach.
Representative images showing immunofluorescence for gastric cell markers and direct reporter fluorescence on cryosections and whole mounts of stomach from 6w Ghrl-Cre;R26-TOM mice. Whole mount stomach shows reporter fluorescence in the corpus and in the pyloric antrum but not in the fundus (a–a’). Although, almost all ghrelin-expressing cells were labeled with TOM, not all TOM+ cells co-localize with ghrelin, indicating ghrelin-expressing cells differentiate into other cell types (b). The tomato reporter is expressed in gastrin-expressing G cells (c), as well as a large number of somatostatin-expressing D cells in the corpus of the stomach (d), but not serotonin-expressing cells (e). All TOM+ cells were also Chromogranin A+, indicative of an endocrine commitment of the ghrelin-expressing cells in the stomach (f). Ghrelin-labeled cells did not colocalize with pepsin C, a marker for chief cells (f), or with DBA, a marker of parietal cell (g). (h) Schematic representation of the lineage tracing experiments defining the fate of ghrelin-labeled cells in the stomach. G, gastrin producing cells; P, parietal cells; EE, enteroendocrine cells; C, chief cells; X/A, ghrelin-producing X/A-like cells; D, somatostatin producing cells. Scale bar, low magnification panels = 50 µm, insets = 20 µm.
Figure 7. Schematic summarizing the lineage tracing experiments to define the fate of ghrelin-labeled cells in the pancreas of Ghrl-Cre;R26-TOM mice.
Ghrelin-expressing cells are rare in the adult pancreas; however cells that formerly expressed ghrelin or their progeny contribute to the final set of endocrine cells in the islet, specifically within the alpha and PP cells. Interestingly, not all α and PP cells are descendants of ghrelin-expressing cells, suggesting that two distinct populations of glucagon- and PP-expressing cells inhabit the adult pancreas. Moreover, we identified Neurog3-independent ghrelin-expressing cells that have the potential to contribute to exocrine and ductal tissue.
Similar articles
- Genetic determinants of pancreatic epsilon-cell development.
Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD, Mellitzer G, Gradwohl G, Serup P. Heller RS, et al. Dev Biol. 2005 Oct 1;286(1):217-24. doi: 10.1016/j.ydbio.2005.06.041. Dev Biol. 2005. PMID: 16122727 - Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development.
Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G, Gradwohl G. Soyer J, et al. Development. 2010 Jan;137(2):203-12. doi: 10.1242/dev.041673. Development. 2010. PMID: 20040487 Free PMC article. - The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration.
Anderson KR, Singer RA, Balderes DA, Hernandez-Lagunas L, Johnson CW, Artinger KB, Sussel L. Anderson KR, et al. Development. 2011 Aug;138(15):3213-24. doi: 10.1242/dev.058693. Development. 2011. PMID: 21750032 Free PMC article. - Development and Characteristics of Pancreatic Epsilon Cells.
Sakata N, Yoshimatsu G, Kodama S. Sakata N, et al. Int J Mol Sci. 2019 Apr 16;20(8):1867. doi: 10.3390/ijms20081867. Int J Mol Sci. 2019. PMID: 31014006 Free PMC article. Review. - Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration.
Rukstalis JM, Habener JF. Rukstalis JM, et al. Islets. 2009 Nov-Dec;1(3):177-84. doi: 10.4161/isl.1.3.9877. Islets. 2009. PMID: 21099270 Review.
Cited by
- Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic α-cells.
Elrick MM, Samson WK, Corbett JA, Salvatori AS, Stein LM, Kolar GR, Naatz A, Yosten GL. Elrick MM, et al. Am J Physiol Regul Integr Comp Physiol. 2016 Jan 15;310(2):R143-55. doi: 10.1152/ajpregu.00369.2014. Epub 2015 Nov 11. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26561648 Free PMC article. - Ghrelin deletion and conditional ghrelin cell ablation increase pancreatic islet size in mice.
Gupta D, Burstein AW, Schwalbe DC, Shankar K, Varshney S, Singh O, Paul S, Ogden SB, Osborne-Lawrence S, Metzger NP, Richard CP, Campbell JN, Zigman JM. Gupta D, et al. J Clin Invest. 2023 Dec 15;133(24):e169349. doi: 10.1172/JCI169349. J Clin Invest. 2023. PMID: 38099492 Free PMC article. - The Spatiotemporal Pattern of Glis3 Expression Indicates a Regulatory Function in Bipotent and Endocrine Progenitors during Early Pancreatic Development and in Beta, PP and Ductal Cells.
Kang HS, Takeda Y, Jeon K, Jetten AM. Kang HS, et al. PLoS One. 2016 Jun 7;11(6):e0157138. doi: 10.1371/journal.pone.0157138. eCollection 2016. PLoS One. 2016. PMID: 27270601 Free PMC article. - β-Cell Heterogeneity and Plasticity.
Yong HJ, Wang YJ. Yong HJ, et al. Adv Anat Embryol Cell Biol. 2024;239:57-90. doi: 10.1007/978-3-031-62232-8_3. Adv Anat Embryol Cell Biol. 2024. PMID: 39283482 Review. - Epigenetic modifications and long noncoding RNAs influence pancreas development and function.
Arnes L, Sussel L. Arnes L, et al. Trends Genet. 2015 Jun;31(6):290-9. doi: 10.1016/j.tig.2015.02.008. Epub 2015 Mar 23. Trends Genet. 2015. PMID: 25812926 Free PMC article. Review.
References
- Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, et al. (2005) Genetic determinants of pancreatic epsilon-cell development. Dev Biol 286: 217–224. - PubMed
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, et al. (2007) Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 12: 457–465. - PubMed
- Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, et al. (2007) An illustrated review of early pancreas development in the mouse. Endocr Rev 28: 685–705. - PubMed
- Pan FC, Wright C (2011) Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 240: 530–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK63608/DK/NIDDK NIH HHS/United States
- DK089523/DK/NIDDK NIH HHS/United States
- U01 DK089523/DK/NIDDK NIH HHS/United States
- DK082590/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- T32 DK007328/DK/NIDDK NIH HHS/United States
- T32 DK07328/DK/NIDDK NIH HHS/United States
- R01 DK082590/DK/NIDDK NIH HHS/United States
- P30 DK063608/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous