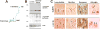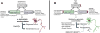The many faces of α-synuclein: from structure and toxicity to therapeutic target - PubMed (original) (raw)
Review
The many faces of α-synuclein: from structure and toxicity to therapeutic target
Hilal A Lashuel et al. Nat Rev Neurosci. 2013 Jan.
Abstract
Disorders characterized by α-synuclein (α-syn) accumulation, Lewy body formation and parkinsonism (and in some cases dementia) are collectively known as Lewy body diseases. The molecular mechanism (or mechanisms) through which α-syn abnormally accumulates and contributes to neurodegeneration in these disorders remains unknown. Here, we provide an overview of current knowledge and prevailing hypotheses regarding the conformational, oligomerization and aggregation states of α-syn and their role in regulating α-syn function in health and disease. Understanding the nature of the various α-syn structures, how they are formed and their relative contributions to α-syn-mediated toxicity may inform future studies aiming to develop therapeutic prevention and intervention.
Figures
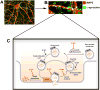
Figure 1. Functional properties of α-synuclein
a, b | Wide field and magnified images of cultured cortical neurons from a postnatal day 1 wild-type mouse showing a neuronal dendrite (as revealed by MAP2 immunostaining; red) opposed to α-synuclein (α-syn)-positive presynaptic densities (green), indicating that α-syn is located in the presynaptic terminals. c | The schematic depicts the various roles of α-syn at the pre-synaptic terminal in the regulation of vesicle trafficking and vesicle refilling (α-syn; blue), as well as the interaction with membrane-associated t-SNARE or the vesicle-associated v-SNARE proteins and neurotransmitter release. Accumulation of α-syn induces an impairment of neurotransmitter release, vesicle recycling and trafficking between synaptic buttons and influence t-SNARE complex assembly stability (α-syn; red), whereas its depletion induces an impairment of vesicle trafficking between the reserve pool and the ready releasable pool and a deficiency in vesicle refilling and neurotransmitter uptake.
Figure 2. Biochemical structure of α-synuclein and its pathological distribution in Parkinson's disease and a mouse model of Lewy body disease
a | Computer-generated model of α-synuclein (α-syn) representing the N-terminal α helices, non-amyloid-β component of Alzheimer's disease amyloid plaques (NAC; depicted in red), and unstructured C-terminal regions. b | Western blot identifying α-syn in brain homogenates from control and Lewy body disease cases that were divided into cytosolic and particulate fractions. α-Syn migrated to 57–60 kDa as well as to 14 kDa in the particulate but not cytosolic fraction due to the different conformational states of the protein. c, d | α-Syn is present in Lewy bodies, neurites, synapses and astroglia in dementia with Lewy bodies (DLB) and Parkinson's disease (PD) and in PDGFβ-human _α_-syn wild type transgenic mice, as indicated by arrows.
Figure 3. Cellular events controlling intracellular α-synuclein levels and possible therapeutic strategies to combat α-synuclein accumulation and transmission
Intracellular α-synuclein (α-syn) levels are tightly regulated by the balance between the rates of α-syn synthesis, clearance and aggregation. a | Abnormalities affecting α-syn synthesis, including SNCA multiplication and polymorphisms, may increase intracellular α-syn levels and induce its accumulation. Accumulation may also be caused by a failure to degrade α-syn. Clearance deficits might arise from failure of the ubiquitin – proteasome system, chaperone-mediated autophagy dysfunction (induced by Parkinson disease-linked mutations) or dysfunction of proteases (neurosin or matrix metalloprotease 9). Finally, certain SNCA mutations, post-translational modifications, oxidative stress, toxins and interaction with oxidized dopamine increase the propensity of α-syn to aggregate and accumulate. b | Targeted mechanisms to reduce α-syn accumulation include decreasing protein synthesis by using Rep1, siRNA or miRNA. Accumulation may also be decreased by activating mechanisms or proteins involved in clearance, such as autophagy, the proteasome, neurosin, MMP9 and heat shock proteins. Additionally, aggregation of α-syn can be decreased using anti-aggregating, antioxidant, or post-translational modification approaches. Finally, immunotherapy may be used to block transmission and oligomer formation.
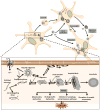
Figure 4. Mechanisms of α-synuclein aggregation and propagation
Unfolded monomers of α-synuclein (α-syn) interact to form two types of dimers: anti-parallel dimers, which do not propagate, and parallel dimmers, which do propagate. A dynamic equilibrium is established between unfolded monomers and both forms of dimers. Interestingly, this process can take place either in the cytoplasm or in association with the cellular membrane. Propagating α-syn dimers can grow by the addition of unfolded monomers and generate ring-like oligomers and oligomers. Ring-like α-syn oligomers interact with the cytoplasmic membrane and form trans-membrane pores, inducing abnormal intracellular calcium influx. Cytoplasmic α-syn oligomers grow by the addition of soluble monomers, forming small amyloid fibrils and then longer fibrils. The accumulation of these amyloid fibrils leads to the formation of intracellular inclusions called Lewy bodies. During α-syn fibrillogenesis and aggregation, the intermediate species (oligomers and amyloid fibrils) are highly toxic, affecting mitochondrial function, endoplasmic reticulum (ER) – Golgi trafficking, protein degradation and/or synaptic transmission. These intracellular effects are thought to induce neurodegeneration. Interestingly, α-syn oligomers and fibrils, as well as the monomers, can be transferred between cells and induce disease spreading to other brain regions. Spreading mechanisms are multiple and can occur via endocytosis, direct penetration, transynaptic transmission, or via membrane receptors.
Similar articles
- Distinct α-Synuclein strains and implications for heterogeneity among α-Synucleinopathies.
Peng C, Gathagan RJ, Lee VM. Peng C, et al. Neurobiol Dis. 2018 Jan;109(Pt B):209-218. doi: 10.1016/j.nbd.2017.07.018. Epub 2017 Jul 24. Neurobiol Dis. 2018. PMID: 28751258 Free PMC article. Review. - Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation.
Uversky VN. Uversky VN. J Neurochem. 2007 Oct;103(1):17-37. doi: 10.1111/j.1471-4159.2007.04764.x. Epub 2007 Jul 10. J Neurochem. 2007. PMID: 17623039 Review. - Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein.
Tsigelny IF, Bar-On P, Sharikov Y, Crews L, Hashimoto M, Miller MA, Keller SH, Platoshyn O, Yuan JX, Masliah E. Tsigelny IF, et al. FEBS J. 2007 Apr;274(7):1862-77. doi: 10.1111/j.1742-4658.2007.05733.x. FEBS J. 2007. PMID: 17381514 - The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration.
Mahul-Mellier AL, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, Leleu M, Knott GW, Lashuel HA. Mahul-Mellier AL, et al. Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):4971-4982. doi: 10.1073/pnas.1913904117. Epub 2020 Feb 19. Proc Natl Acad Sci U S A. 2020. PMID: 32075919 Free PMC article. - Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies.
Villar-Piqué A, Lopes da Fonseca T, Outeiro TF. Villar-Piqué A, et al. J Neurochem. 2016 Oct;139 Suppl 1:240-255. doi: 10.1111/jnc.13249. Epub 2015 Sep 11. J Neurochem. 2016. PMID: 26190401 Review.
Cited by
- Olfactory Dysfunction in Parkinson's Disease, Its Functional and Neuroanatomical Correlates.
Torres-Pasillas G, Chi-Castañeda D, Carrillo-Castilla P, Marín G, Hernández-Aguilar ME, Aranda-Abreu GE, Manzo J, García LI. Torres-Pasillas G, et al. NeuroSci. 2023 Jun 5;4(2):134-151. doi: 10.3390/neurosci4020013. eCollection 2023 Jun. NeuroSci. 2023. PMID: 39483318 Free PMC article. Review. - Inhibition of amyloid-β plaque formation by α-synuclein.
Bachhuber T, Katzmarski N, McCarter JF, Loreth D, Tahirovic S, Kamp F, Abou-Ajram C, Nuscher B, Serrano-Pozo A, Müller A, Prinz M, Steiner H, Hyman BT, Haass C, Meyer-Luehmann M. Bachhuber T, et al. Nat Med. 2015 Jul;21(7):802-7. doi: 10.1038/nm.3885. Epub 2015 Jun 22. Nat Med. 2015. PMID: 26099047 - Identification of Two Novel Peptides That Inhibit α-Synuclein Toxicity and Aggregation.
Popova B, Wang D, Rajavel A, Dhamotharan K, Lázaro DF, Gerke J, Uhrig JF, Hoppert M, Outeiro TF, Braus GH. Popova B, et al. Front Mol Neurosci. 2021 Apr 12;14:659926. doi: 10.3389/fnmol.2021.659926. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33912013 Free PMC article. - MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration.
Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. Harms AS, et al. J Neurosci. 2013 Jun 5;33(23):9592-600. doi: 10.1523/JNEUROSCI.5610-12.2013. J Neurosci. 2013. PMID: 23739956 Free PMC article. - The hot sites of α-synuclein in amyloid fibril formation.
Khammari A, Arab SS, Ejtehadi MR. Khammari A, et al. Sci Rep. 2020 Jul 22;10(1):12175. doi: 10.1038/s41598-020-68887-2. Sci Rep. 2020. PMID: 32699326 Free PMC article.
References
- McKeith IG, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. - PubMed
- Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000;247 Suppl 2:II3–II10. Detailed description of the pathoanatomy that occurs in Parkinson's disease. - PubMed
- Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. - PubMed
- Chartier-Harlin MC, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. - PubMed
- Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 NS076411/NS/NINDS NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- NS044233/NS/NINDS NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous